Abstract
Background
Vaccination against SARS-CoV-2 is recommended for cancer patients. However, long-term data on the effectiveness in the pediatric setting are lacking.
Methods
Pediatric patients < 18 years on active treatment for cancer and without prior SARS-CoV-2 infection received three doses of an mRNA vaccine. The clinical course and humoral and cellular immunity were evaluated at the end of the follow-up period of ≥ 1 year after the third dose of vaccine.
Results
SARS-CoV-2 infection occurred in 17 of 19 analyzed patients (median age 16.5 years) during the follow-up period (median 17 months), but no severe symptoms were seen. At ≥ 1 year after the last SARS-CoV-2 antigen exposure, 4 of 17 patients had received the recommended booster vaccine. At the end of the follow-up period, all evaluable 15 patients had anti-SARS-CoV-2 receptor-binding domain IgG antibodies. Twelve of the 15 patients had neutralizing antibody titers ≥ 1:10 against the Delta variant and 12/15 and 13/15 against the BA.1 and BA.5 variants, respectively. Specific T cells against SARS-CoV-2 antigens were seen in 9/13 patients.
Conclusions
Most SARS-CoV-2-vaccinated pediatric cancer patients had SARS-CoV-2 infections and limited interest in booster vaccination. At 1 year after the last antigen exposure, which was mostly an infection, humoral immune responses remained strong.
Trial registration
German Clinical Trials Register DRKS00025254, May 26, 2021.
Similar content being viewed by others
Introduction
It has become clear that children and adolescents receiving therapy for cancer or undergoing hematopoietic cell transplantation are at an increased risk for severe or even lethal infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [1, 2]. Whereas the rapid development of vaccines against SARS-CoV-2 and the successful implementation of vaccine programs resulted in the decrease in morbidity and mortality in risk groups such as elderly individuals or in immunocompromised adults [3, 4]; there is limited information on the effectiveness of SARS-CoV-2 vaccination in pediatric patients on active cancer treatment, and data on the long-term follow up are lacking [5,6,7]. We recently reported on the results of a prospective longitudinal study in 21 pediatric patients receiving chemotherapy for cancer which demonstrated that 3 doses of SARS-CoV-2 mRNA vaccine resulted in both humoral and cellular immunity in most patients [6]. Here, we present the follow-up of these patients for at least 1 year after the third dose of the vaccine and evaluate both the clinical course as well as the humoral and cellular immune responses to SARS-CoV-2.
Patients and methods
Immunocompromised pediatric cancer patients up to 18 years of age, who were on active treatment for any malignancy, were eligible to be enrolled in the study. As previously described in detail [6], exclusion criteria were previous or ongoing infections with SARS-CoV-2, pregnancy, and primary immunodeficiency. Patients were vaccinated with the mRNA vaccine BNT162b2 (Comirnaty, BioNTech/Pfizer), which was administered preferentially at lymphocyte counts ≥ 1000 cells/µl. Two doses of the vaccine were given within 3–6 weeks, followed by a booster vaccination between 4 weeks and 6 months after the second vaccination, which in some cases was delayed due to cancer treatment or complications of therapy. Approximately 2 weeks after the booster vaccine, the immune response was assessed, an interim analysis was performed, and results were reported [6]. The follow-up period started with the immune response assessment after the third dose of vaccine and was scheduled for at least 1 year. The follow-up period ended with the evaluation of the occurrence and severity of SARS-CoV-2 infections and the assessment of a complete blood count; lymphocyte subsets; immunoglobulin G (IgG) level; antibodies against the receptor-binding domain (RBD) and the nucleocapsid antigen of SARS-CoV-2, neutralizing antibodies against the Delta variant and the Omicron variants BA.1 and BA.5; SARS-CoV-2-specific T cells; and antigen-specific memory B cells. The severity of infection with SARS-CoV-2 was classified by the score given by Dong et al. [8]. As previously described in detail, antibodies against the RBD of SARS-CoV-2 and the nucleocapsid antigen were assessed using the Abbott Alinity I platform (Abbott Laboratories, Abbott Park, Illinois) and neutralizing antibodies in an authentic virus-neutralizing assay [9]. SARS-CoV-2-specific T cells were examined by an ELISPOT assay [T-SPOT COVID (Oxford Immunotec)] using two different SARS-CoV-2-specific antigens [10]. The antigen pools contained peptides of the S1 subunit and RBD of the spike protein and peptides of the nucleocapsid protein, respectively [10]. Antigen-specific memory B cells were assessed by ELISPOT using peripheral blood mononuclear cells (PBMCs) of the participants activated for 5 or 6 days with R848 (1 µg/ml) and 96-well Multiscreen-IP filter plates (Millipore, Merck KGaA), which were coated with recombinant SARS-CoV nucleocapsid protein (NP)- maltose-binding fusion protein (MBP) (2 µg/ml), SARS-CoV-2 RBD (1 µg/ml), influenza virus NP-MBP (2 µg/ml) and tetanus toxoid (5 µg/ml, lot 317,490, GSK Vaccines). The total concentration of antibody-secreting B cells was assessed using anti-IgG coated with mouse anti-human IgG mAb (clone MT91/145; Mabtech AB) [11].
Differences between groups (e.g., dose of vaccine) were analyzed using the Wilcoxon rank test for paired samples. Differences between patients with solid tumors and hematological malignancies were assessed with the Mann–Whitney test. A p-value (2-tailed) of < 0.05 was considered statistically significant. Analyses were performed using GraphPad Prism software version 5.0.2 (Graph Pad Software, San Diego, California).
The study was approved by the local Ethical Committees Frankfurt (2021–128), Münster (2021–467-b-S), and Freiburg (2021–1382) and was performed in accordance with the Declaration of Helsinki. The study was registered with the German Registry for Clinical Trials (DRKS00025254). All patients and caregivers provided written informed consent.
Results
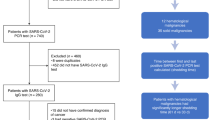
The follow-up period after the third dose of the SARS-CoV-2 vaccine was evaluated in a total of 19 pediatric patients [11 female, 8 male; median age at study entry (range) 16.5 years (13.2–17.9)] (Table 1). One of the 21 patients originally enrolled in the study died of the malignancy during the study, and one patient withdrew his consent. The patients suffered from hematological malignancies (n = 13) or solid tumors (n = 6). The median length (range) of the follow-up period was 17 (12–21) months. At the beginning of the follow-up (e.g., after the third dose of the SARS-CoV-2 vaccine), two patients received intensive chemotherapy, 13 maintenance chemotherapy, and four patients were already off therapy. At the end of the follow-up period (defined by the final clinical and laboratory evaluation), none of the patients received intensive chemotherapy, five patients were on maintenance therapy, and 14 were off therapy (Table 1).
Clinical course during follow-up
Two out of the 19 patients received a fourth dose of the SARS-CoV-2 vaccine (second booster vaccination) (Table 1). While none of the patients had evidence of infection with SARS-CoV-2 up to the third dose, 17 of the 19 patients had at least one SARS-CoV-2 infection during the follow-up period (16 patients with one infection, one patient (#1) with two infections; positive PCR tests in 15 and positive antigen tests in 3 episodes of infection, respectively). Two patients had an asymptomatic infection (score 1), and 16 infections were associated with mild upper respiratory or gastrointestinal symptoms (score 2). No patient experienced moderate, severe, or critical symptoms (scores 3, 4, and 5). Two patients (#12 and #17) received specific immunoglobulins for COVID-19.
Laboratory evaluation
Four patients withdrew their consent for an additional blood draw at the end of the follow-up period. The blood samples were drawn at a median of 17 months (12–21) after the third dose of the SARS-CoV-2 vaccine.
The median number (range) of lymphocytes (n = 11) was 1587/µl (711–2710), of CD4+ T cells 542/µl (337–1200; no patient with counts below the normal value of 300/µl), and of CD19+ cells 259/µl (14–510; 3 patients with counts below the normal value of 100/µl). The median (range) of total IgG (n = 10) was 854 mg/dl (700–1393).
All 15 patients evaluated had sustained measurable anti-SARS-CoV-2 receptor-binding domain (RBD) IgG antibodies as a marker of immunity (Table 2). Seventeen patients experienced a SARS-CoV-2 infection at a median (range) of 10 (1–15) months prior to the immunological assessment at the end of the follow-up. The geometric mean of anti-SARS-CoV-2 RBD IgG antibodies was 2463.8 BAU/ml, which was higher than after the first (3.8), second (179.9), and third dose of vaccine (1032.3) (Fig. 1). The geometric mean anti-RBD-IgG titer of patients with a hematological malignancy was lower than in those with a solid tumor (2023 and 2305 BAU/ml, respectively), but this difference was not statistically significant (p = 0.8581). The two patients without SARS-CoV 2 infection had an RBD IgG titer of 4011.5 BAU/ml (this patient (#14) had received a 4th dose of vaccine) and 296.1 BAU/ml (this patient (#6) had received 3 doses of vaccine), respectively. Seven out of 13 patients (54%) with confirmed infection with SARS-CoV-2 after the third dose of vaccine revealed measurable anti-SARS-CoV-2 nucleocapsid antibodies (Table 2). Compared to the beginning of the follow-up period (approximately two weeks after the third dose of SARS-CoV-2 vaccine), patients had significantly higher mean neutralizing antibody titers against both the Delta variant of SARS-CoV-2 and the BA.1 variant (Fig. 2). Twelve out of 15 evaluated patients had neutralizing antibodies with a titer of at least 1:10 against the Delta and BA.1 variant and 14 out of 15 against the BA.5 variant. The mean titer against both BA.1 and BA.5 variants was 1:80 (Fig. 2).
Longitudinal results of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) anti-receptor-binding domain immunoglobulin G (IgG) test. Data points from individual study participants are connected. Differences between groups were assessed using the Wilcoxon matched-pairs signed-rank test. BAU binding antibody units, IgG immunoglobulin G; RBD receptor-binding domain. ***p < .001, the result is statistically significant
Neutralizing titers against the Delta variant of SARS-CoV-2 after the first, second, and third vaccine doses, as well as at the end of the follow-up period (left), and neutralizing titers against the Omicron variant BA.1 (center) and BA.5 variant (right) after the third dose and/or at the end of the follow-up period. The horizontal line indicates the median titer. Differences between groups were assessed using the Wilcoxon matched-pairs signed-rank test. **p < .01; ***p < .001, results are statistically significant
At the end of the follow-up period, one out of the 13 patients evaluated showed specific T cells against both the spike and the nucleoprotein, 8 patients showed specific T cells against the spike, but not against the nucleoprotein, whereas in 4 patients, no specific T cells against any of these proteins were detected (Table 2). T-cell responses did not correlate with the humoral response against SARS-CoV-2 (data not shown). Memory B-cell analysis was performed on three of the participants (#5, #9, and #11). At the end of the follow-up period, all of them revealed vigorous memory B cell responses against the SARS CoV-2 RBD and low levels against the SARS CoV-2 nucleoprotein (data not shown).
Discussion
In a prospective longitudinal study of 21 pediatric patients receiving chemotherapy for cancer, we recently demonstrated that 3 doses of a SARS-CoV-2 vaccine resulted in both humoral and cellular immunity in most of the patients [6]. Here, we present the follow-up of these patients at least 1 year after the third dose of vaccine and evaluate the clinical course, SARS-CoV-2 antibody titers, and specific T cell and memory B cell responses.
The Standing Vaccination Commission (STIKO) of Germany recommends 3 exposures to SARS-CoV-2 antigens (either vaccination or infection) for immunocompromised pediatric and adult patients which should consist of at least two doses of vaccine and an additional booster vaccine after a period of ≥ 12 months [12]. In our study, only two out of the 19 patients received a 4th dose of vaccine. In contrast, 13 out of 17 patients had not received a booster vaccine at 1 year after the last SARS-CoV-2 antigen exposure, which was a SARS-CoV-2 infection in 12 patients and a vaccination in one patient. Although it was speculated that there will be a high rate of acceptance of the SARS-CoV-2 booster vaccination in individuals at increased risk for severe infections [12], data in immunocompromised patients are lacking to date. Our results in immunocompromised pediatric patients reflect the findings of a large population-based panel study performed at the end of 2021 which reported that a considerable proportion of fully vaccinated adults hesitate to receive a booster dose of the SARS-CoV-2 vaccine [13].
At the beginning of 2022, the strict regulations that meant to avoid SARS-CoV-2 infections—such as lockdowns or wearing a face mask—were considerably relaxed in Germany, which most likely accounts for the fact that the vast majority of SARS-CoV-2 infections in our study population occurred during the first 6 months of 2022 with a median (range) of 4 months (0–13) after the third dose of vaccine. Whereas a prospective study of 230 cancer patients who received a third dose of the SARS-CoV-2 vaccine as a booster vaccination reported that in their participants the serological titer cut-off below 803 BAU/ml was predictive of breakthrough infection [14], we found that 9 out of 17 infections occurred in patients with a titer > 803 BAU/ml. In addition, in a study of 2686 adults with varying immune-suppressive diseases who had received two doses of the SARS-CoV-2 vaccine, about 10% of patients had a severe course of the infection or died, and impaired serological and T cell responses were associated with severe infection [15]. In our study population, however, the infections caused mild symptoms at most, which is not surprising, as 90% of our patients had detectable antibody titers after the third dose of vaccine which might have provided protection from severe disease [6].
In 15 patients of our patient population, the serological response to SARS-CoV-2 was assessed after a median (range) of 10 (1–15) months after the last antigen exposure which was a SARS-CoV-2 infection in 13 patients. All patients demonstrated sustained measurable anti-SARS-CoV-2 RBD IgG antibodies as a marker of immunity. In contrast, only 54% of patients with a known infection after the third vaccine dose had measurable anti-SARS-CoV-2 nucleocapsid antibodies, suggesting that this test might not reliably rule out a prior infection.
A total of 12 patients had neutralizing antibodies with a titer of at least 1:10 against the Delta and BA.1 variants of SARS-CoV-2 and 13 against BA.5. Notably, after the BA.1 variant had been the most prevalent variant in Germany during the first half of 2022, the BA.5 variant became the dominant variant (https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Virologische_Basisdaten.html;jsessionid=127DD66D7D1E0E47EEEC5E0F07246600.internet052?nn=13490888#doc14716546bodyText5).
Our results are novel, as data on immune responses to SARS-CoV-2 vaccinations in pediatric immunocompromised patients are scarce, in particular, regarding data on long-term follow-up [5,6,7]. Corroborating our results reported previously [6], one retrospective study reported on good efficacy of SARS-CoV-2 vaccination in 13 patients (median age 17 years) receiving chemotherapy for a solid tumor [7]. Donze et al. [5] evaluated the anti-SARS-CoV-2 immunity in 38 pediatric cancer patients and assessed the humoral, but not the cellular immune response. At 259 days post-vaccination, the probability of maintaining immunity against SARS-CoV-2 declined to 50% in the 15/38 patients who acquired post-vaccination immunity during the first 3 months after vaccination [5]. In comparison, our study shows a sustained humoral response at the end of the follow-up period which can be explained by the fact that we did not remove patients with a documented SARS-CoV-2 infection, as this reflects the real-life setting. In contrast, we observed that 4 out of 13 patients had no detectable anti-SARS-CoV-2 T cells; although, after the second dose of the SARS-CoV-2 vaccine, specific T cells had been demonstrated in two of them [6]. Data in adults suffering from lung cancer showed that the T cell immune response against SARS-CoV-2 at 6 and 12 months after the third dose of vaccine did not significantly differ, but these patients received immunotherapy for their cancer [16].
We recognize that our study population is small, although our analysis included the largest number of reported pediatric cancer patients receiving the recommended three doses of vaccine. In addition, due to a change in SARS-CoV-2 variants, the clinical severity of a SARS-CoV-2 infection has been alleviated considerably since the beginning of 2021, and there is no proof that the course of infection would have been more severe without the vaccination. Notably, our real-life analysis includes the assessment of both humoral and cellular immunity against SARS-CoV-2 and followed the patients for more than 1 year after the third dose of the vaccine. Nevertheless, larger series are mandatory to confirm our data and to monitor the results with emerging variants of SARS-CoV-2 and will be the basis for future recommendations for vaccination against SARS-CoV-2 in immunocompromised patients.
In conclusion, our findings show that despite repeated consultations the majority of pediatric cancer patients have currently limited interest in booster vaccinations against SARS-CoV-2. The results suggest that infections with SARS-CoV-2 are common in these patients and lead to detectable humoral immune response after one year of the last antigen exposure, including neutralizing antibodies against the dominant variant at the time of infection.
Data availability
All data and materials are available from the authors on request.
References
Haeusler GM, Ammann RA, Carlesse F, Groll AH, Averbuch D, Castagnola E, Agyeman PKA, Phillips B, Gilli F, Solopova G, Attarbaschi A, Wegehaupt O, Speckmann C, Sung L, Lehrnbecher T (2021) SARS-CoV-2 in children with cancer or after haematopoietic stem cell transplant: an analysis of 131 patients. Eur J Cancer 159:78–86
Mukkada S, Bhakta N, Chantada GL, Chen Y, Vedaraju Y, Faughnan L, Homsi MR, Muniz-Talavera H, Ranadive R, Metzger M, Friedrich P, Agulnik A, Jeha S, Lam C, Dalvi R, Hessissen L, Moreira DC, Santana VM, Sullivan M, Bouffet E, Caniza MA, Devidas M, Pritchard-Jones K, Rodriguez-Galindo C, Global Registry of C-iCC (2021) Global characteristics and outcomes of SARS-CoV-2 infection in children and adolescents with cancer (GRCCC): a cohort study. Lancet Oncol 22:1416–1426
Surie D, DeCuir J, Zhu Y, Gaglani M, Ginde AA, Douin DJ, Talbot HK, Casey JD, Mohr NM, Zepeski A, McNeal T, Ghamande S, Gibbs KW, Files DC, Hager DN, Ali H, Taghizadeh L, Gong MN, Mohamed A, Johnson NJ, Steingrub JS, Peltan ID, Brown SM, Martin ET, Khan A, Bender WS, Duggal A, Wilson JG, Qadir N, Chang SY, Mallow C, Kwon JH, Exline MC, Lauring AS, Shapiro NI, Columbus C, Halasa N, Chappell JD, Grijalva CG, Rice TW, Stubblefield WB, Baughman A, Womack KN, Rhoads JP, Hart KW, Swan SA, Lewis NM, McMorrow ML, Self WH, Network IVY (2022) Early estimates of bivalent mRNA vaccine effectiveness in preventing COVID-19-associated hospitalization among immunocompetent adults aged ≥65 years - IVY network, 18 states, September 8-November 30, 2022. MMWR Morb Mortal Wkly Rep 71:1625–1630
Tenforde MW, Patel MM, Gaglani M, Ginde AA, Douin DJ, Talbot HK, Casey JD, Mohr NM, Zepeski A, McNeal T, Ghamande S, Gibbs KW, Files DC, Hager DN, Shehu A, Prekker ME, Erickson HL, Gong MN, Mohamed A, Johnson NJ, Srinivasan V, Steingrub JS, Peltan ID, Brown SM, Martin ET, Monto AS, Khan A, Hough CL, Busse LW, Duggal A, Wilson JG, Qadir N, Chang SY, Mallow C, Rivas C, Babcock HM, Kwon JH, Exline MC, Botros M, Lauring AS, Shapiro NI, Halasa N, Chappell JD, Grijalva CG, Rice TW, Jones ID, Stubblefield WB, Baughman A, Womack KN, Rhoads JP, Lindsell CJ, Hart KW, Zhu Y, Naioti EA, Adams K, Lewis NM, Surie D, McMorrow ML, Self WH, Network IVY (2022) Effectiveness of a third dose of Pfizer-BioNTech and Moderna vaccines in preventing COVID-19 hospitalization among immunocompetent and immunocompromised adults - United States, August-December 2021. MMWR Morb Mortal Wkly Rep 71:118–124
Donze C, Min V, Ninove L, de Lamballerie X, Revon Riviere G, Verschuur A, Saultier P, Andre N (2023) BNT162b2 COVID-19 vaccines in children, adolescents and young adults with cancer-A 1-year follow-up. Vaccines (Basel) 11:989
Lehrnbecher T, Sack U, Speckmann C, Groll AH, Boldt A, Siebald B, Hettmer S, Demmerath EM, Reemtsma J, Schenk B, Ciesek S, Klusmann JH, Jassoy C, Hoehl S (2023) Longitudinal immune response to 3 doses of messenger RNA vaccine against coronavirus disease 2019 (COVID-19) in pediatric patients receiving chemotherapy for cancer. Clin Infect Dis 76:e510–e513
Revon-Riviere G, Ninove L, Min V, Rome A, Coze C, Verschuur A, de Lamballerie X, Andre N (2021) The BNT162b2 mRNA COVID-19 vaccine in adolescents and young adults with cancer: a monocentric experience. Eur J Cancer 154:30–34
Dong Y, Mo X, Hu Y, Qi X, Jiang F, Jiang Z, Tong S (2020) Epidemiology of COVID-19 among children in china. Pediatrics 145:e20200702
Delbruck M, Hoehl S, Toptan T, Schenk B, Grikscheit K, Metzler M, Herrmann E, Ciesek S (2022) Low but recoverable markers of humoral immune response to BNT162b2 in elderly LTCF residents five to seven months after two-dose vaccination. Front Aging 3:883724
Kruse M, Dark C, Aspden M, Cochrane D, Competiello R, Peltz M, Torres L, Wrighton-Smith P, Dudek M (2021) Performance of the T-SPOT().COVID test for detecting SARS-CoV-2-responsive T cells. Int J Infect Dis 113:155–161
Kannenberg J, Trawinski H, Henschler R, Buhmann R, Honemann M, Jassoy C (2022) Antibody course and memory B-cell response in the first year after SARS-CoV-2 infection. J Infect Dis 226:664–672
Piechotta VJK, Berner R, Bogdan C, Burchard G, Heininger U, Hummers E, von Kries R, Littmann MTL, Meerpohl J, Röbl-Mathieu MTM, van der Sande M, Sander LE, Terhardt M, Kü SV-B, Ow SW, Wiedermann-Schmidt U, Widders G, Zepp F (2023) Empfehlung der STIKO zur Implementierung der COVID-19-Impfung in die Empfehlungen der STIKO 2023 und die dazugehörige wissenschaftliche Begründung. Epid Bul 21:7–48
Paul E, Fancourt D (2022) Predictors of uncertainty and unwillingness to receive the COVID-19 booster vaccine: an observational study of 22,139 fully vaccinated adults in the UK. Lancet Reg Health Eur 14:100317
Nelli F, Fabbri A, Virtuoso A, Giannarelli D, Giron Berrios JR, Marrucci E, Fiore C, Schirripa M, Signorelli C, Chilelli MG, Primi F, Pessina G, Natoni F, Silvestri MA, Ruggeri EM (2023) Effects of antibody response after booster vaccination on SARS-CoV-2 breakthrough infections and disease outcomes in advanced cancer patients: a prospective analysis of the vax-on-third study. Curr Oncol 30:5103–5115
Barnes E, Goodyear CS, Willicombe M, Gaskell C, Siebert S, IdS T, Murray SM, Rea D, Snowden JA, Carroll M, Pirrie S, Bowden SJ, Dunachie SJ, Richter A, Lim Z, Satsangi J, Cook G, Pope A, Hughes A, Harrison M, Lim SH, Miller P, Klenerman P, Basu N, Gilmour A, Irwin S, Meacham G, Marjot T, Dimitriadis S, Kelleher P, Prendecki M, Clarke C, Mortimer P, McIntyre S, Selby R, Meardon N, Nguyen D, Tipton T, Longet S, Laidlaw S, Orchard K, Ireland G, Thomas D, Kearns P, Kirkham A, McInnes IB, consortium P, Consensus, Group OC (2023) SARS-CoV-2-specific immune responses and clinical outcomes after COVID-19 vaccination in patients with immune-suppressive disease. Nat Med 29:1760–1774
Lasagna A, Cassaniti I, Arena F, Bergami F, Percivalle E, Comolli G, Sarasini A, Ferrari A, Cicognini D, Schiavo R, Lo Cascio G, Pedrazzoli P, Baldanti F (2023) Persistence of immune response elicited by three doses of mRNA vaccine against SARS-CoV-2 in a cohort of patients with solid tumors: a one-year follow-up. Int J Mol Sci 24:6731
Acknowledgements
We would like to thank Daniel Jarisch, Institute of Medical Virology, Goethe University Frankfurt, for conducting the neutralization assays.
Funding
Open Access funding enabled and organized by Projekt DEAL. This study was supported in part by the Goethe-Corona-Fonds of the University of Frankfurt.
Author information
Authors and Affiliations
Contributions
Design of the study: Benjamin Siebald, Andreas Boldt, Christian Jassoy, Sebastian Hoehl, Thomas Lehrnbecher. Data acquisition: Benjamin Siebald, Andreas H. Groll, Sarah Salou. Experimental data: Andreas Boldt, Sabine Seiffert, Ulrich Sack, Judith Reemtsma, Christian Jassoy, Sebastian Hoehl. Analyzing data: Benjamin Siebald, Andreas Boldt, Christian Jassoy, Sebastian Hoehl, Thomas Lehrnbecher. Interpretation of the data: all authors. Writing of manuscript: Benjamin Siebald, Andreas Boldt, Christian Jassoy, Sebastian Hoehl, Thomas Lehrnbecher. Reviewing manuscript: all autors. Final approval of mauscript: all authors.
Corresponding author
Ethics declarations
Ethical approval
The study was approved by the local Ethical Committees Frankfurt (2021–128), Münster (2021–467-b-S), and Freiburg (2021–1382), and was performed in accordance with the Declaration of Helsinki. All patients and caregivers provided written informed consent.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Sebastian Hoehl and Thomas Lehrnbecher equally share senior authorship.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Siebald, B., Groll, A.H., Salou, S. et al. Pediatric cancer patients vaccinated against SARS-CoV-2—a clinical and laboratory follow-up. Support Care Cancer 32, 221 (2024). https://doi.org/10.1007/s00520-024-08422-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00520-024-08422-5