Abstract
Purpose
There are numerous guidelines that recommend physical activity (PA) in people diagnosed with cancer, but the quality of these guidelines is unknown. The aim of this study was to identify existing PA guidelines for cancer survivors, describe the recommendations, and assess their methodology quality.
Methods
A rapid review of the literature was conducted in PubMed and EMBASE, supplemented by a search of the grey literature. The methodological quality of the guidelines was assessed using the AGREE II checklist. A descriptive synthesis of the recommendations from guidelines judged to be of good quality has been performed.
Results
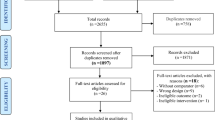
A total of nine guidelines published between 2006 and 2019 were included. Of nine guidelines, five achieved a high enough AGREE II score and were judged to be of good quality for use in clinical practice. We found that the recommendations from the five guidelines converged on the prescription of supervised PA (aerobic and resistance exercise) of at least 75 min per week of high intensity or 150 min per week of moderate intensity, spread over two to five sessions per week, equating to a PA dose between 8.70 and 17.5 MET.h/week. The recommendations were applicable to address the most common side effects of cancer and its treatment, namely fatigue, lymphedema, anxiety, depressive symptoms, health-related quality of life (QoL), survival, and physical function. However, no guideline recommends PA to improve other cancer-related outcomes, such as cognitive impairment, falls, sexual function, and peripheral neuropathy frequently experienced by cancer survivors. No guideline also referred to work outcomes (i.e., work ability, return to work, etc.).
Conclusion
Most PA guidelines for cancer survivors are of good quality. However, specific PA guidelines are needed for a given cancer site (e.g., location, stage), at a particular phase of the cancer trajectory, and for specific outcomes including return to work (RTW) in order to tailor PA to each cancer survivor.


Similar content being viewed by others
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Guo YJ, Tang J, Li JM, Zhu LL, Xu JS (2021) Exploration of interventions to enhance return-to-work for cancer patients: a scoping review. Clin Rehabil 35(12):1674–1693
Spence RR, Heesch KC, Brown WJ (2010) Exercise and cancer rehabilitation: a systematic review. Cancer Treat Rev 36(2):185–194
Rizzo A (2016) The role of exercise and rehabilitation in the cancer care plan. J Adv Pract Oncol 7(3):339–342
Lowe SS, Watanabe SM, Courneya KS (2009) Physical activity as a supportive care intervention in palliative cancer patients: a systematic review. J Support Oncol 7(1):27–34
Torregrosa C, Chorin F, Beltran EEM, Neuzillet C, Cardot-Ruffino V (2022) Physical activity as the best supportive care in cancer: the clinician’s and the researcher’s perspectives. Cancers. 14(21):5402
Loprinzi PD, Cardinal BJ (2012) Effects of physical activity on common side effects of breast cancer treatment. Breast Cancer 19(1):4–10
Fong DYT, Ho JWC, Hui BPH, Lee AM, Macfarlane DJ, Leung SSK et al (2012) Physical activity for cancer survivors: meta-analysis of randomised controlled trials. BMJ. 344(jan30 5):e70
Speck RM, Courneya KS, Mâsse LC, Duval S, Schmitz KH (2010) An update of controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. J Cancer Surviv 4(2):87–100
Knols R, Aaronson NK, Uebelhart D, Fransen J, Aufdemkampe G (2005) Physical exercise in cancer patients during and after medical treatment: a systematic review of randomized and controlled clinical trials. JCO. 23(16):3830–3842
Schmitz KH, Courneya KS, Matthews C, Demark-Wahnefried W, Galvão DA, Pinto BM et al (2010) American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc 42(7):1409–1426
Rock CL, Doyle C, Demark-Wahnefried W, Meyerhardt J, Courneya KS, Schwartz AL et al (2012) Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin 62(4):243–274
Segal R, Zwaal C, Green E, Tomasone JR, Loblaw A, Petrella T (2017) Exercise for people with cancer: a clinical practice guideline. Curr Oncol 24(1):40–46
Zopf EM, Baumann FT, Pfeifer K (2014) Physical activity and exercise recommendations for cancer patients during rehabilitation. Rehabilitation (Stuttg) 53(1):2–7
Field MJ, Lohr KN (1990) Clinical practice guidelines: directions for a new program [Internet]. National Academies Press, Washington, D.C. Cited 2023 Aug 18. Available from: https://www.nap.edu/catalog/1626
Graham R, Mancher M, Miller Wolman D, Greenfield S, Steinberg E (2011) Clinical practice guidelines we can trust [Internet]. National Academies Press (US), Washington (DC) Cited 2023 May 12. Available from: http://www.ncbi.nlm.nih.gov/books/NBK209539/
Woolf SH, Grol R, Hutchinson A, Eccles M, Grimshaw J (1999) Potential benefits, limitations, and harms of clinical guidelines. BMJ. 318(7182):527–530
Murad MH (2017) Clinical practice guidelines: a primer on development and dissemination. Mayo Clin Proc 92(3):423–433
Guerra-Farfan E, Garcia-Sanchez Y, Jornet-Gibert M, Nuñez JH, Balaguer-Castro M, Madden K (2023) Clinical practice guidelines: the good, the bad, and the ugly. Injury. 54:S26–S29
Franco JVA, Arancibia M, Meza N, Madrid E, Kopitowski K (2020) Clinical practice guidelines: concepts, limitations and challenges. Medwave. 20(3):e7887
Shallwani SM, King J, Thomas R, Thevenot O, Angelis GD, Aburub AS et al (2019) Methodological quality of clinical practice guidelines with physical activity recommendations for people diagnosed with cancer: a systematic critical appraisal using the AGREE II tool. PLoS One 14(4):e0214846
Mickan S, Burls A, Glasziou P (2011) Patterns of “leakage” in the utilisation of clinical guidelines: a systematic review. Postgrad Med J 87(1032):670–679
Wilson TN, Nambiema A, Porro B, Descatha A, Aublet-Cuvelier A, Evanoff B et al (2023) Effectiveness of physical activity interventions on return to work after a cancer diagnosis: a systematic review and meta-analysis. J Occup Rehabil 33(1):4–19
Ancellin R, Gaillot-de SJ (2017) Bénéfices de l’activité physique pendant et après cancer : des connaissances scientifiques aux repères pratiques. Oncologie. 19(3):95–107
Van Waart H, Stuiver MM, van Harten WH, Geleijn E, Kieffer JM, Buffart LM et al (2015) Effect of low-intensity physical activity and moderate- to high-intensity physical exercise during adjuvant chemotherapy on physical fitness, fatigue, and chemotherapy completion rates: results of the PACES randomized clinical trial. J Clin Oncol 33(17):1918–1927
Mijwel S, Jervaeus A, Bolam KA, Norrbom J, Bergh J, Rundqvist H et al (2019) High-intensity exercise during chemotherapy induces beneficial effects 12 months into breast cancer survivorship. J Cancer Surviv 13(2):244–256
Thijs KM, de Boer AGEM, Vreugdenhil G, van de Wouw AJ, Houterman S, Schep G (2012) Rehabilitation using high-intensity physical training and long-term return-to-work in cancer survivors. J Occup Rehabil 22(2):220–229
Berglund G, Bolund C, Gustafsson UL, Sjödén PO (1994) One-year follow-up of the “Starting Again” group rehabilitation programme for cancer patients. Eur J Cancer 30A(12):1744–1751
Burgio KL, Goode PS, Urban DA, Umlauf MG, Locher JL, Bueschen A et al (2006) Preoperative biofeedback assisted behavioral training to decrease post-prostatectomy incontinence: a randomized, controlled trial. J Urol 175(1):196–201 discussion 201
Ibrahim M, Muanza T, Smirnow N, Sateren W, Fournier B, Kavan P et al (2017) Time course of upper limb function and return-to-work post-radiotherapy in young adults with breast cancer: a pilot randomized control trial on effects of targeted exercise program. J Cancer Surviv 11(6):791–799
Rogers LQ, Hopkins-Price P, Vicari S, Pamenter R, Courneya KS, Markwell S et al (2009) A randomized trial to increase physical activity in breast cancer survivors. Med Sci Sports Exerc 41(4):935–946
Garritty C, Gartlehner G, Nussbaumer-Streit B, King VJ, Hamel C, Kamel C et al (2021) Cochrane Rapid Reviews Methods Group offers evidence-informed guidance to conduct rapid reviews. J Clin Epidemiol 130:13–22
Khangura S, Konnyu K, Cushman R, Grimshaw J, Moher D (2012) Evidence summaries: the evolution of a rapid review approach. Syst Rev 1(1):10
Hamel C, Michaud A, Thuku M, Skidmore B, Stevens A, Nussbaumer-Streit B et al (2021) Defining Rapid Reviews: a systematic scoping review and thematic analysis of definitions and defining characteristics of rapid reviews. J Clin Epidemiol 129:74–85
Babineau J (2014) Product Review: Covidence (Systematic Review Software). J Can Health Libr Assoc 35(2):68–71
Brouwers MC, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G et al (2010) AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ. 182(18):E839–E842
AGREE Collaboration (2003) Development and validation of an international appraisal instrument for assessing the quality of clinical practice guidelines: the AGREE project. Qual Saf Health Care 12(1):18–23
Brouwers MC, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G et al (2010) Development of the AGREE II, part 2: assessment of validity of items and tools to support application. CMAJ. 182(10):E472–E478
Désy F. Revue systématique de l’évaluation de la qualité et du contenu scientifique des guides de pratique. 2018, Cited 10 May 2022; Available from: https://papyrus.bib.umontreal.ca/xmlui/handle/1866/20809
Brouwers MC, Kerkvliet K, Spithoff K, Consortium ANS (2016) The AGREE Reporting Checklist: a tool to improve reporting of clinical practice guidelines. BMJ. 352:i1152
NIH. About Systematic Evidence Reviews and Clinical Practice Guidelines | NHLBI, NIH [Internet]. 2011. Cited 10 May 2022, Available from: https://www.nhlbi.nih.gov/node/80397
AGREE II rater concordance calculator-mcmaster-university [Internet]. Cited 11 May 2022, Available from: https://image4.slideserve.com/7912200/agree-ii-rater-concordance-calculator-mcmaster-university-l.jpg
Smith CAM, Toupin-April K, Jutai JW, Duffy CM, Rahman P, Cavallo S et al (2015) A systematic critical appraisal of clinical practice guidelines in juvenile idiopathic arthritis using the Appraisal of Guidelines for Research and Evaluation II (AGREE II) Instrument. PLoS One 10(9):e0137180
de GRC MC, Romano-Lieber NS, Ribeiro E, de Melo DO (2019) Comparison of the methodological quality and transparency of Brazilian practice guidelines. Ciênc Saúde Coletiva 24:3947–3956
Campbell KL, Winters-Stone KM, Wiskemann J, May AM, Schwartz AL, Courneya KS et al (2019) Exercise guidelines for cancer survivors: consensus statement from International Multidisciplinary Roundtable. Med Sci Sports Exerc 51(11):2375–2390
HAS. HAS 2019. Prescription d’activité physique et sportive. Cancers: sein, colorectal, prostate. [Internet]. 2019, cited 30 May 2022. Available from: https://institut-recapps.com/has-2019-prescription-dactivite-physique-et-sportive-cancers-sein-colorectal-prostate-juillet-2019/
Hayes SC, Newton RU, Spence RR, Galvão DA (2019) The Exercise and Sports Science Australia position statement: exercise medicine in cancer management. J Sci Med Sport 22(11):1175–1199
Cormie P, Atkinson M, Bucci L, Cust A, Eakin E, Hayes S et al (2018) Clinical Oncology Society of Australia position statement on exercise in cancer care. Med J Aust 209(4):184–187
Brunet J, Sabiston CM, Meterissian S (2012) Physical activity and breast cancer survivorship: evidence-based recommendations. Am J Lifestyle Med 6(3):224–240
McNeely ML, Peddle CJ, Parliament M, Courneya KS (2006) Cancer rehabilitation: recommendations for integrating exercise programming in the clinical practice setting. Curr Cancer Ther Rev 2(4):351–360
Van den Berg JP, Velthuis MJ, Gijsen BCM, Lindeman E, van der Pol MA, Hillen HFP et al (2011) Guideline “Cancer rehabilitation”. Ned Tijdschr Geneeskd 155(51):A4104
Silver JK (2014) Cancer rehabilitation and prehabilitation may reduce disability and early retirement. Cancer. 120(14):2072–2076
Siedler MR, Lamadrid P, Humphries MN, Mustafa RA, Falck-Ytter Y, Dahm P et al (2021) The quality of physical activity guidelines, but not the specificity of their recommendations, has improved over time: a systematic review and critical appraisal. Appl Physiol Nutr Metab 46(1):34–45
Qaseem A, Forland F, Macbeth F, Ollenschläger G, Phillips S, van der Wees P et al (2012) Guidelines International Network: toward international standards for clinical practice guidelines. Ann Intern Med 156(7):525–531
Kastner M, Bhattacharyya O, Hayden L, Makarski J, Estey E, Durocher L et al (2015) Guideline uptake is influenced by six implementability domains for creating and communicating guidelines: a realist review. J Clin Epidemiol 68(5):498–509
Gagliardi AR, Brouwers MC, Palda VA, Lemieux-Charles L, Grimshaw JM (2011) How can we improve guideline use? A conceptual framework of implementability. Implement Sci 6(1):26
Pomey MP, Flora L, Karazivan P, Dumez V, Lebel P, Vanier MC et al (2015) Le « Montreal model » : enjeux du partenariat relationnel entre patients et professionnels de la santé. Santé Publique S1:41
Buffart LM, Galvão DA, Brug J, Chinapaw MJM, Newton RU (2014) Evidence-based physical activity guidelines for cancer survivors: current guidelines, knowledge gaps and future research directions. Cancer Treat Rev 40(2):327–340
Stein KD, Syrjala KL, Andrykowski MA (2008) Physical and psychological long-term and late effects of cancer. Cancer. 112(11 Suppl):2577–2592
de Boer AGEM, de Wind A, Coenen P, van Ommen F, Greidanus MA, Zegers AD et al (2023) Cancer survivors and adverse work outcomes: associated factors and supportive interventions. Br Med Bull 145(1):60–71
Rinaldi Y. Sport et cancer. POST’U 2016 – Paris [Internet]. 2016; Cited 11 Jul 2022, Available from: https://www.fmcgastro.org/textes-postus/postu-2016-paris/sport-et-cancer/
Carroll JE, Small BJ, Tometich D, Zhai W, Zhou X, Luta G et al (2019) Sleep disturbance and neurocognitive outcomes in older breast cancer patients: interaction with genotype. Cancer. 125(24):4516–4524
Funding
This manuscript was prepared as part of the SIRIC ILIAD program funded by the French National Cancer Institute (INCa), the French Ministry of Health, and the Institute of Health and Medical Research (Inserm), INCa-DGOS-INSERM-ITMO Cancer_18011.
Têtê Norbert Wilson received a scholarship from the Togolese government and the non-profit organization Grain de Sel Togo, Inc., for his Ph.D. research.
Author information
Authors and Affiliations
Contributions
TNW: designed and planned the study, developed the search strategy, screened guidelines for eligibility, extracted and analyzed the data, interpreted the results, and drafted the manuscript. YR, BE, AAC: designed and planned the study, coordinated all stages of the study, critically revised the methodological and scientific aspects of the manuscript. BP: designed and planned the study, contributed to the quality assessment of guidelines, critically revised the methodological and scientific aspects of the manuscript. All authors read and approved the final manuscript.”
Corresponding author
Ethics declarations
Ethical approval and consent to participate
Ethical approval and patient consent are not required for this study as it is based on secondary data and does not directly involve human participants. The study was conducted in accordance with the ethical standards of the National Consultative Ethics Committee, the 1964 Declaration of Helsinki, and its subsequent amendments or comparable ethical standards.
Competing interests
The authors have no conflicts of interest to disclose in relation to the content of this article.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 97 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wilson, T.N., Roquelaure, Y., Evanoff, B. et al. Physical activity in people diagnosed with cancer: a rapid review of recommendations and critical appraisal of international guidelines. Support Care Cancer 31, 679 (2023). https://doi.org/10.1007/s00520-023-08123-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00520-023-08123-5