Abstract
Background
Chemotherapy-induced peripheral neuropathy (CIPN) is a common side effect of chemotherapy, especially after taxane-based therapy. This study aimed to examine the relationship between symptoms of anxiety and depression before the start of taxane-based chemotherapy and the development of CIPN in women with breast cancer.
Methods
In this prospective study, women with breast cancer receiving taxane-based (neo)adjuvant chemotherapy were recruited from four hospitals in the Netherlands. Patients completed questionnaires assessing anxiety and depressive symptoms before treatment and CIPN before treatment (T0), 6 weeks after start of treatment (T1), after the last cycle of chemotherapy (T2), and 6 months after the end of treatment (T3). Mixed model analyses were used to investigate whether medium/high levels of anxiety or depression at baseline are associated with the level of CIPN during and after treatment.
Results
Among the 61 participating women, 14 (23%) reported medium/high levels of anxiety and 29 (47.5%) reported medium/high levels of depressive symptoms at baseline. The group of women with medium/high baseline levels of anxiety showed a significantly higher increase in CIPN during and after chemotherapy than women with low baseline levels of anxiety (p < .001). No relationship between depressive symptoms at baseline and the development of CIPN was found.
Conclusion
This study showed that baseline medium to high levels of anxiety but not depressive symptoms impacted the development of CIPN during and in the 6 months after treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Chemotherapy has contributed significantly to the increased survival rates in women with breast cancer. However, chemotherapy, especially taxane-based, may have long lasting adverse side effects such as peripheral neuropathy (CIPN). CIPN is characterized by symptoms such as numbness, tingling, pins and needles sensation, hyperalgesia or allodynia, and burning pain in the hands or feet in a stocking-glove distribution [1,2,3]. CIPN can negatively affect daily functioning and quality of life [4] and may impact optimal treatment [5, 6]. To date, there are no effective interventions or agents available to prevent or treat CIPN [7]. Consequently, dose reduction or chemotherapy cessation may be necessary to prevent severe CIPN [8,9,10].
The reported prevalence of CIPN in cancer patients treated with taxane-based chemotherapy differs from 11 to 87% [11]. Also, the variability in CIPN severity and duration is considerable, ranging from mild to severe and from temporal to chronic. CIPN generally develops during chemotherapy and may persist after treatment. The variations in intensity, development, and duration are not well understood and are likely to be multifactorial by nature [12]. Characteristics such as age, BMI, diabetes, preexisting peripheral neuropathy, and the influence of cumulative dose are found to increase the risk of developing CIPN [4, 13, 14]. Besides these sociodemographic and clinical characteristics, psychological factors may play a role as well. Previous research pointed to an association between anxiety and CIPN in cancer survivors after chemotherapy [5, 15]. Lee and colleagues (2018) found in their prospective observational study among women with breast cancer that pre-treatment anxiety but not depressive symptoms was associated with elevated neuropathic symptoms directly after ending treatment, and this effect persisted after 8 months. A limitation of this study was that no data were available of CIPN during treatment and therefore it remains unclear when differences in CIPN start to occur. Moreover, in this study, no validated method to assess CIPN was used.
The primary aim of the present study was to investigate the relationship between symptoms of anxiety and depression and the level and development of CIPN during and after taxane-based (neo)adjuvant chemotherapy in women with breast cancer. Nonindependence of the scores of individual participants over time were taken into account using mixed model analyses. We hypothesized that the increase of CIPN during and after chemotherapy is enhanced in patients who report medium to high levels of anxiety before the start of taxane-based chemotherapy. No relationship between symptoms of depression and CIPN was expected.
Methods
Study design and patient population
Participants were enrolled in this prospective observational study in four hospitals in the Netherlands (Leiden University Medical Centre, Groene Hart Hospital, Haga Hospital, and Alrijne Hospital) between January 2020 and September 2021. Participants were women with breast cancer, aged 18 years or older, treated with taxane-based (neo)adjuvant chemotherapy. Exclusion criteria were obvious cognitive impairments and not speaking Dutch well enough to participate in the assessments. Eligible participants completed baseline questionnaires before the first cycle of chemotherapy, recording socio-demographic as well as symptoms of anxiety and depression. CIPN was measured before start of chemotherapy (T0), after 6 weeks (T1), after the last cycle of chemotherapy (after 15 weeks, T2), and 6 months after ending chemotherapy (T3). Clinical data (i.e., cancer grade, chemotherapy regimen, discontinuation of treatment) were extracted from patients’ medical records. The NUMBNESS study was approved by the certified Medical Ethical Committee Leiden-Den Haag-Delft (METC-LDD, registration number: N19.111). Written or digital informed consents were obtained from all patients.
Sociodemographic and clinical characteristics
Before the start of chemotherapy, information was provided on sociodemographic characteristics such as age, marital status, household composition, education level, weight and height, smoking, and alcohol use by self-report questionnaires. Also information was obtained on the presence of diabetes or rheumatism, use of antidepressants, and having received chemotherapy in the past. Tumor stage (TNM staging system), type of taxane regiment, and information about discontinuing the chemotherapy were obtained from patients’ medical files.
Primary outcome: neuropathic symptoms
The European Organization for Research and Treatment of Cancer Quality of life Questionnaire Chemotherapy-Induced Peripheral Neuropathy 20 (EORTC QLQ-CIPN20) was used to assess CIPN. Patients were asked at four time points to indicate how often they had experienced peripheral neuropathic symptoms in the past week. Nineteen items were answered on a four-point Likert scale ranging from (1) not at all to (4) very much. Item 20 is a male-related question and was not included in the questionnaire. Item 19 can only be answered by patients who can drive a car. Total scores of the CIPN20 are used and range from 18 to 76, with higher scores indicating more neuropathy. Cronbach’s alpha of the EORTC QLQ-CIPN20 at the four time points was 0.88 (T0), 0.75 (T1), 0.87 (T2), and 0.95 (T3).
Secondary dependent variable: symptoms of anxiety and depression
Anxiety was measured by the Generalized Anxiety Disorder (GAD-7) [16]. The GAD-7 contains 7 items assessing core anxiety symptoms. Patients rate their frequency of symptoms on a 4-point Likert scale ranging from 0 (not at all) to 3 (almost every day) over the previous 2 weeks. GAD-7 scores can range from 0 to 21, with higher scores indicating higher anxiety symptomatology. The cut-off point for the presence of moderately/high anxiety symptoms is 5 [17]. Cronbach’s alpha of the GAD-7 was 0.86.
Depression was measured by the Patient Health Questionnaire (PHQ-9). The PHQ-9 contains 9 items to score the symptoms of depression on a 4-point Likert scale ranging from 0 (not at all) to 3 (almost every day) over the previous 2 weeks. The total PHQ-9 scores can range from 0 to 27, with higher scores indicating more depression symptoms. The cut-off point for the presence of moderately severe depressive symptoms is 5 [18]. Cronbach’s alpha of the PHQ-9 was 0.76.
Statistical analysis
Baseline characteristics were determined and associated with CIPN using Pearson’s product correlation coefficients, independent sample t-test, or one-way ANOVA analyses. Variables significantly (p < .05) associated with CIPN at baseline were used as covariates in the main analyses. Moreover, when baseline anxiety or depression is associated with baseline CIPN, baseline CIPN will be included as covariate in the main analyses. By controlling for the baseline CIPN levels, it is possible to investigate the effect of baseline anxiety or depression on the developing CIPN during and after treatment, independent of the CIPN baseline levels.
Mixed models with maximum likelihood estimation and an unstructured covariance matrix with a 2-level structure (time-level and patient-level) were used. Mixed model analyses allow the number of observations per assessment to differ and therefore missing data were not imputed. A sequence of models was fitted to investigate the development of CIPN during and in the first 6 months after chemotherapy and whether this is influenced by the level of anxious or depressive symptoms at baseline. First, a model with no explanatory variables, only the intercept (i.e., an unconditional model), was calculated to determine the amount of variance at the person and time level. Second, a model with only time as explanatory variable (unconditional growth model) was calculated to determine whether CIPN changed from pre-treatment till 6 months after ending chemotherapy. Intercept and time are entered both as fixed and random effects as each subject may have its own unique intercept and slope. Third, the unconditional growth model was extended into a conditional growth model by including (i) possible covariates (i.e., age, BMI, receiving chemotherapy in past) and baseline CIPN, (ii) baseline level of anxiety or depression, and (iii) the interaction term of time by level of anxiety or depression as fixed effect. By including the interaction term, we were able to investigate whether CIPN develops differently over time depending on baseline levels of anxiety and depression. Regression estimates and 95% confidence interval of fixed effects and the Akaike information criteria (AIC) are presented. A model-based graph was created as an aid to determine how the relationship between anxiety and depressive symptoms and CIPN could be understood.
Results
Patients’ sociodemographic and clinical characteristics
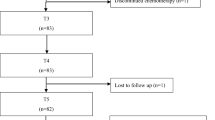
In total, 63 eligible patients were included. Two patients were excluded from further analyses because they did not finish the chemotherapy due to an allergic reaction. Of the remaining 61 women, 59 completed assessment 6 weeks after starting chemotherapy (T1), 56 after the last cycle of chemotherapy (T2), and 36 completed assessment 6 months after ending chemotherapy (T3). Table 1 shows baseline clinical and demographic characteristics. At inclusion, mean age was 51.7 years (SD 10.4; range 25.0–75.0) and mean BMI was 26.4 (SD 4.9; range 19.9–42.1).
Nine percent of the patients were diagnosed with stage II disease and most patients received treatment with weekly paclitaxel (84%, n=51). Sixteen patients (26.2%) had received treatment with chemotherapy in the past, all other than taxane based. Baseline characteristics such age, BMI, smoking, diabetes, rheumatism, and chemotherapy in the past were not found to be associated with CIPN levels before start chemotherapy. At baseline, 23% (n=14) of the women reported moderately/high anxiety symptoms and 48.4% (n=31) reported moderately/high depressive symptoms. Baseline anxiety was found to be associated with baseline CIPN (p = .001). That is, patients with a low anxiety score had a baseline CIPN score of 21.1 (SD = 3.17) and high anxiety score had baseline CIPN score of 25.93 (SD = 8.28). No association was found between baseline depression and baseline CIPN (p =.07).
Association between CIPN, time, and baseline level of anxiety and depression
Intraclass correlation coefficients of the unconditional model showed that 43% of the variance was at the person level and the remaining 57% was at the time level. These results indicate that scores on CIPN differed enough between patients and over time to justify a two-level model (AIC = 1398.59). Next, we computed an unconditional growth model by entering time into the model. This model fitted the data better than the unconditional model (AIC = 1351.60) and showed that CIPN increases over time from a mean score of 22.25 at T0 (95% CI 20.58–23.92) to 28.46 at T3 (95% CI 26.48–30.45). Moreover, the conditional growth model with time, level of anxiety at baseline, and the interaction term time by anxiety was computed. This model fitted the data better than the unconditional growth model (AIC = 1300.01). No baseline characteristics such as BMI, age, smoking, and diabetes were included as these were found not to be significantly associated with baseline CIPN. This model (see Table 2) shows that time, baseline CIPN, and baseline level of anxiety had an impact on level of CIPN.
The mean difference in CIPN between patients scoring low and medium/high on baseline anxiety was 3.52 (p =.002). Moreover, also the development of CIPN over time was found to differ between patients scoring low and medium/high on baseline anxiety (see also Figure 1).
Similarly, we computed a conditional growth model with baseline level of depression. This model did fit the data better than the unconditional growth model (AIC = 1327.55) but showed that level of depression did not impact the development (p = .89) of CIPN (see also Figure 2).
Discussion
This prospective study investigated the relationship between levels of anxiety and depression before the start of chemotherapy and the development and level of CIPN during and after treatment. Our results indicate that pre-treatment anxiety but not depressive symptoms may be a risk factor for developing CIPN in women with breast cancer during and after treatment with taxane-based (neo)adjuvant chemotherapy. These results are in line with Lee’s study that also found an association between pre-treatment anxiety and CIPN [15]. The present study adds to this finding by showing that more anxious patients experience more neuropathy before start and during chemotherapy and that, 6 months after ending chemotherapy, neuropathic symptoms increase even further. In contrast, less anxious patients start at a lower level of neuropathy and although showing an increase in neuropathic symptoms during chemotherapy this level is much lower than in the more anxious group and does not increase after ending chemotherapy.
Both psychological and biological effects of anxiety need to be considered when explaining the association between anxiety and the development of CIPN. Studies have shown that people with high levels of anxiety may be hypersusceptible to pain [19, 20], partly because they are more inclined to catastrophic thinking [21, 22]. Catastrophic thinking is characterized by attention to threat, overemphasis of the probability of a catastrophic outcome, and rumination about the worst possible consequences. It seems to play a unique role in the experience of higher levels of physical disability and negative physical sensations, like pain [23, 24], because it focuses the attention on the pain. This focus on pain may even be exacerbated when being informed by the medical specialist about the possible side effects of chemotherapy such as neuropathic symptoms. More anxious patients might be more suggestible to the negative expectations about getting pain (nocebo effect) which may function as a self-fulfilling prophesy [25, 26]. In addition to psychological mechanisms, biological factors may explain the relationship between anxiety and the development of more CIPN. More anxious patients may exhibit higher levels of proinflammatory cytokines interleukin-6 (IL-6) [27,28,29], which might interfere with recovery from the nerve injury in CIPN, resulting in more CIPN over time [30, 31]. Interestingly, in this study as well as in the study by Lee (2018), no relationship between depressive symptoms and the development of neuropathy was found. More research into the underlying mechanisms is needed to examine why pretreatment depression is not associated with CIPN severity and development while it has been related to more physical pain in other populations [32].
Neuropathy is a common adverse effect of taxane-based chemotherapy, but also of other anticancer drugs and may last for months and even years [33]. Given the increasing number of cancer survivors, there is an urgent need for prevention and treatment strategies for patients with CIPN. The burden experienced by neuropathy may impact optimal cancer treatment by limiting the dose of the anticancer drugs or discontinuing the treatment [2] and may worsen global quality of life and physical, role, cognitive, and social functioning compared to survivors without CIPN [34, 35]. To date, no treatment can be proposed as a gold standard to prevent or treat CIPN [7, 33, 36]. The results of this study might open the door for studies investigating interventions that may help prevent the development of excessive neuropathy by reducing pre-treatment anxiety. Till date, most psychological interventions focus on reducing or dealing with neuropathy after it developed [34, 37]. Whether psychological and/or pharmacological interventions with the aim of reducing anxiety before chemotherapy may have a positive impact on the development of CIPN should be investigated in future studies.
This study has several strengths such as the use of validated measures for CIPN, anxiety, and depression. For the degree of CIPN, we have used the EORTC-QLQ-CIPN20. This measure has a strong association with the often used NCI-CTCAE [38]. While both measures are validated, the QLQ-CIPN20 questionnaire provides more detailed information, distinguishes more subtle degrees of neuropathy, and is more responsive to change over time [4]. To detect subtle changes in level of neuropathy is especially important in the case of prevention. Moreover, CIPN was assessed four times in a 9-month period. Data was analyzed using mixed model analyses which are an extension of a linear regression model with the advantage that it focuses on individual patient’s patterns of scores through time rather than on mean values at each of the time points. A mixed model acknowledges that differences at later time points may be due to baseline effects and that the four separate scores over time by the same subject are correlated. A limitation of the present study is the relatively small sample size and short follow-up period. CIPN may develop months to years after ending chemotherapy [4, 15, 39]. Long-term research is required to investigate whether the effect of pre-treatment anxiety on the development of CIPN remains.
In conclusion, the level and persistence of CIPN after taxane-based chemotherapy seem to be associated with preexisting anxiety. Consequently, future studies may investigate the mechanisms by which anxiety influences CIPN and whether interventions targeting pre-treatment anxiety could lessen the development of CIPN.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Mustafa Ali M, Moeller M, Rybicki L, Moore HCF (2017) Long-term peripheral neuropathy symptoms in breast cancer survivors. Breast Cancer Res Treat 166(2):519–526
Staff NP, Grisold A, Grisold W, Windebank AJ (2017) Chemotherapy-induced peripheral neuropathy: a current review. Ann Neurol 81(6):772–781
Brewer JR, Morrison G, Dolan ME, Fleming GF (2016) Chemotherapy-induced peripheral neuropathy: current status and progress. Gynecol Oncol 140(1):176–183
Eckhoff L, Knoop A, Jensen MB, Ewertz M (2015) Persistence of docetaxel-induced neuropathy and impact on quality of life among breast cancer survivors. Eur J Cancer 51(3):292–300
Miaskowski C, Paul SM, Mastick J, Abrams G, Topp K, Smoot B, Kober KM, Chesney M, Mazor M, Mausisa G, Schumacher M, Conley YP, Sabes JH, Cheung S, Wallhagen M, Levine JD (2018) Associations between perceived stress and chemotherapy-induced peripheral neuropathy and otoxicity in adult cancer survivors. J Pain Symptom Manag 56(1):88–97
Bonhof CS, Mols F, Vos MC, Pijnenborg JMA, Boll D, Vreugdenhil G, Ezendam NPM, van de Poll-Franse LV (2018) Course of chemotherapy-induced peripheral neuropathy and its impact on health-related quality of life among ovarian cancer patients: a longitudinal study. Gynecol Oncol 149(3):455–463
Hu LY, Mi WL, Wu GC, Wang YQ, Mao-Ying QL (2019) Prevention and treatment for chemotherapy-induced peripheral neuropathy: therapies based on CIPN mechanisms. Curr Neuropharmacol 17(2):184–196
Bhatnagar B, Gilmore S, Goloubeva O, Pelser C, Medeiros M, Chumsri S, Tkaczuk K, Edelman M, Bao T (2014) Chemotherapy dose reduction due to chemotherapy induced peripheral neuropathy in breast cancer patients receiving chemotherapy in the neoadjuvant or adjuvant settings: a single-center experience. Springerplus 3:366
Miltenburg NC, Boogerd W (2014) Chemotherapy-induced neuropathy: a comprehensive survey. Cancer Treat Rev 40(7):872–882
Lyman GH (2009) Impact of chemotherapy dose intensity on cancer patient outcomes. J Natl Compr Cancer Netw 7(1):99–108
Banach M, Juranek JK, Zygulska AL (2017) Chemotherapy-induced neuropathies-a growing problem for patients and health care providers. Brain Behav 7(1):e00558
Rivera DR, Ganz PA, Weyrich MS, Bandos H, Melnikow J (2018) Chemotherapy-associated peripheral neuropathy in patients with early-stage breast cancer: a systematic review. J Natl Cancer Inst 110(2):131–140
Bao T, Basal C, Seluzicki C, Li SQ, Seidman AD, Mao JJ (2016) Long-term chemotherapy-induced peripheral neuropathy among breast cancer survivors: prevalence, risk factors, and fall risk. Breast Cancer Res Treat 159(2):327–333
Beijers AJ, Mols F, Tjan-Heijnen VC, Faber CG, van de Poll-Franse LV, Vreugdenhil G (2015) Peripheral neuropathy in colorectal cancer survivors: the influence of oxaliplatin administration. Results from the population-based PROFILES registry. Acta Oncol 54(4):463–469
Lee KM, Jung D, Hwang H, Son KL, Kim TY, Im SA, Lee KH, Hahm BJ (2018) Pre-treatment anxiety is associated with persistent chemotherapy-induced peripheral neuropathy in women treated with neoadjuvant chemotherapy for breast cancer. J Psychosom Res 108:14–19
Esser P, Hartung TJ, Friedrich M, Johansen C, Wittchen HU, Faller H, Koch U, Harter M, Keller M, Schulz H, Wegscheider K, Weis J, Mehnert A (2018) The Generalized Anxiety Disorder Screener (GAD-7) and the anxiety module of the Hospital and Depression Scale (HADS-A) as screening tools for generalized anxiety disorder among cancer patients. Psychooncology 27(6):1509–1516
Johnson SU, Ulvenes PG, Oktedalen T, Hoffart A (2019) Psychometric properties of the general anxiety disorder 7-item (GAD-7) scale in a heterogeneous psychiatric sample. Front Psychol 10:1713
Kroenke K, Spitzer RL, Williams JB (2001) The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 16(9):606–613
Keogh E, Cochrane M (2002) Anxiety sensitivity, cognitive biases, and the experience of pain. J Pain 3(4):320–329
Thompson T, Keogh E, French CC, Davis R (2008) Anxiety sensitivity and pain: generalisability across noxious stimuli. Pain 134(1-2):187–196
Meints SM, Mawla I, Napadow V, Kong J, Gerber J, Chan ST, Wasan AD, Kaptchuk TJ, McDonnell C, Carriere J, Rosen B, Gollub RL, Edwards RR (2019) The relationship between catastrophizing and altered pain sensitivity in patients with chronic low-back pain. Pain 160(4):833–843
Terry S, Dalban C, Rioux Leclercq N, Adam J, Meylan M, Buart S, Bougouin A, Lespagnol A, Dugay F, Colina Moreno I, Lacroix G, Lorens JB, Gausdal G, Fridman WH, Mami-Chouaib F, Chaput N, Beuselinck B, Chabaud S, Barros Monteiro J et al (2021) Association of AXL and PD-L1 expression with clinical outcomes in patients with advanced renal cell carcinoma treated with PD-1 blockade. Clin Cancer Res:6749–6760
Grant DM, Beck JG (2010) What predicts the trajectory of rumination?: a prospective evaluation. J Anxiety Disord 24(5):480–486
Edwards RR, Kronfli T, Haythornthwaite JA, Smith MT, McGuire L, Page GG (2008) Association of catastrophizing with interleukin-6 responses to acute pain. Pain 140(1):135–144
Blasini M, Corsi N, Klinger R, Colloca L (2017) Nocebo and pain: an overview of the psychoneurobiological mechanisms. Pain Rep 2(2):1–9
Colloca L, Benedetti F (2007) Nocebo hyperalgesia: how anxiety is turned into pain. Curr Opin Anaesthesiol 20(5):435–439
O’Donovan A, Hughes BM, Slavich GM, Lynch L, Cronin MT, O’Farrelly C, Malone KM (2010) Clinical anxiety, cortisol and interleukin-6: evidence for specificity in emotion-biology relationships. Brain Behav Immun 24(7):1074–1077
Lazaridou A, Martel MO, Cahalan CM, Cornelius MC, Franceschelli O, Campbell CM, Haythornthwaite JA, Smith M, Riley J, Edwards RR (2018) The impact of anxiety and catastrophizing on interleukin-6 responses to acute painful stress. J Pain Res 11:637–647
Murphy TM, O’Donovan A, Mullins N, O’Farrelly C, McCann A, Malone K (2015) Anxiety is associated with higher levels of global DNA methylation and altered expression of epigenetic and interleukin-6 genes. Psychiatr Genet 25(2):71–78
Starkweather A (2010) Increased interleukin-6 activity associated with painful chemotherapy-induced peripheral neuropathy in women after breast cancer treatment. Nurs Res Pract 2010:281531
Zhou YQ, Liu Z, Liu ZH, Chen SP, Li M, Shahveranov A, Ye DW, Tian YK (2016) Interleukin-6: an emerging regulator of pathological pain. J Neuroinflammation 13(1):141
de Heer EW, Gerrits MM, Beekman AT, Dekker J, van Marwijk HW, de Waal MW, Spinhoven P, Penninx BW, van der Feltz-Cornelis CM (2014) The association of depression and anxiety with pain: a study from NESDA. PLoS One 9(10):e106907
Poupon L, Kerckhove N, Vein J, Lamoine S, Authier N, Busserolles J, Balayssac D (2015) Minimizing chemotherapy-induced peripheral neuropathy: preclinical and clinical development of new perspectives. Expert Opin Drug Saf 14(8):1269–1282
Poulin PA, Romanow HC, Rahbari N, Small R, Smyth CE, Hatchard T, Solomon BK, Song X, Harris CA, Kowal J, Nathan HJ, Wilson KG (2016) The relationship between mindfulness, pain intensity, pain catastrophizing, depression, and quality of life among cancer survivors living with chronic neuropathic pain. Support Care Cancer 24(10):4167–4175
Bonhof CS, Trompetter HR, Vreugdenhil G, van de Poll-Franse LV, Mols F (2020) Painful and non-painful chemotherapy-induced peripheral neuropathy and quality of life in colorectal cancer survivors: results from the population-based PROFILES registry. Support Care Cancer 28(12):5933–5941
Li Y, Lustberg MB, Hu S (2021) Emerging pharmacological and non-pharmacological therapeutics for prevention and treatment of chemotherapy-induced peripheral neuropathy. Cancers (Basel) 13(4):766
Smith AM, Leeming A, Fang Z, Hatchard T, Mioduszewski O, Schneider MA, Ferdossifard A, Shergill Y, Khoo EL, Poulin P (2021) Mindfulness-based stress reduction alters brain activity for breast cancer survivors with chronic neuropathic pain: preliminary evidence from resting-state fMRI. J Cancer Surviv 15(4):518–525
Weitkamp K, Romer G, Rosenthal S, Wiegand-Grefe S, Daniels J (2010) German Screen for Child Anxiety Related Emotional Disorders (SCARED): reliability, validity, and cross-informant agreement in a clinical sample. Child Adolesc Psychiatry Ment Health 4:19
Osmani K, Vignes S, Aissi M, Wade F, Milani P, Levy BI, Kubis N (2012) Taxane-induced peripheral neuropathy has good long-term prognosis: a 1- to 13-year evaluation. J Neurol 259(9):1936–1943
Author information
Authors and Affiliations
Contributions
RV, MT, and CH have made substantial contributions to the conception, design, and analysis. RV, MT, CH, NW, HO, MC, JP, and JK have contributed to the inclusion, interpretation of the data, draft, and revision of the manuscript. All authors have approved the submitted version and have agreed both to be personally accountable for the author’s own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the certified Medical Ethical Committee Leiden-Den Haag-Delft (METC-LDD, registration number: N19.111).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Verhoeff-Jahja, R., ter Kuile, M.M., Weijl, N.I. et al. Symptoms of anxiety but not depression before start of taxane-based chemotherapy are associated with peripheral neuropathy: a multicenter study in women with breast cancer. Support Care Cancer 30, 6947–6953 (2022). https://doi.org/10.1007/s00520-022-07093-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-022-07093-4