Abstract
Context
In 2011, a multidisciplinary palliative team (MPT) was established at Rigshospitalet (DK) and a cross-sectional study in inpatients was carried out at the Departments of Oncology and Hematology. High symptom burden, high prevalence of pain (64%), and insufficient analgesic treatment were demonstrated. In 2019, a similar study was carried out.
Objectives
This study compares prevalence of symptoms including pain and analyzes analgesic treatment of adult in-patients in a comprehensive cancer center.
Methods
Two cross-sectional studies (May–Jun 2011; Feb–Sep 2019). Inclusion criteria: malignant diseases, age ≥ 18 y, able to understand Danish. EORTC QLQ-C30 and Brief Pain Inventory (BPI) were applied.
Results
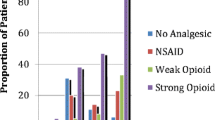
A total of 134 and 183 inpatients were included in 2011 and 2019, respectively. Differences in the two populations were seen; in 2019 more patients had advanced disease (P = 0.0096), lower performance status (P = 0.0028), and a palliative treatment plan (P = 0.0034). The prevalence of impairments and symptoms was high and similar in the 2 years with exception of severe pain (P = 0.0143) and neuropathic pain (P < 0.0001) which increased in 2019. Moreover, pain relief significantly improved, and significantly fewer patients with pain were left untreated. Significant increase in opioid and adjuvant analgesic prescription in 2019.
Conclusion
An overall unchanged high symptom burden was observed. However, improvement of pain management was observed in 2019. The establishment of a MPT may possibly have contributed to improved pain management.


Similar content being viewed by others
Data Availability
Data is available upon request and can be given within the approval from the Danish Data Protection Agency (VD-2019–68).
Code availability
N/A.
References
Jordhøy MS, Fayers P, Loge JH, Ahlner-Elmqvist M, Kaasa S (2001) Quality of life in palliative cancer care: results from a cluster randomized trial. J Clin Oncol 19:3884–3894
Nordly M et al (2019) Systematic fast-track transition from oncological treatment to dyadic specialized palliative home care: DOMUS — a randomized clinical trial. Palliat Med 33(2):135–149
El-Jawahri A, LeBlanc T, VanDusen H, Traeger L, Greer JA, Pirl WF, Jackson VA, Telles J, Rhodes A, Spitzer TR, McAfee S, Chen YA, Lee SS, Temel JS (2016) Effect of inpatient palliative care on quality of life 2 weeks after hematopoietic stem cell transplantation: a randomized clinical trial. JAMA 316(20):2094–2103. https://doi.org/10.1001/jama.2016.16786
Ferrell BR, Temel JS, Temin S, Alesi ER, Balboni TA, Basch EM, Firn JI, Paice JA, Peppercorn JM, Phillips T, Stovall EL, Zimmermann C, Smith TJ (2017) Integration of palliative care into standard oncology care: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol 35(1):96–112. https://doi.org/10.1200/JCO.2016.70.1474
Jordan K, Aapro M, Kaasa S et al (2018) European Society for Medical Oncology (ESMO) position paper on supportive and palliative care. Ann Oncol 29:36–43
Kaasa S, Loge JH, Aapro M et al (2018) Integration of oncology and palliative care: a Lancet Oncology Commission. Lancet Oncol 19:e588–e653. https://doi.org/10.1016/S1470-2045(18)30415-7
Kurita GP, Tange UB, Farholt H, Sonne NM, Strömgren AS, Ankersen L, Kristensen L, Bendixen L, Grønvold M, Petersen MA, Nordly M, Christrup L, Niemann C, Sjøgren P (2013) Pain characteristics and management of inpatients admitted to a comprehensive cancer centre: a cross-sectional study. Acta Anaesthesiol Scand 57:518–525
Strömgren AS, Niemann CU, Tange UB et al (2014) Quality of life and symptoms in patients with malignant diseases admitted to a comprehensive cancer centre. Support Care Cancer 22:1843–1849
Pourhoseingholi MA, Vahedi M, Rahimzadeh M (2013) Sample size calculation in medical studies. Gastroenterol Hepatol Bed Bench 6(1):14–17
Naing L, Winn T, Rusli BN (2006) Practical issues in calculating the sample size for prevalence studies. Arch Orofac Sci 1:9–14
Daniel WW (1999) Biostatistics: A Foundation for Analysis in the Health Sciences 7th edition
Thrusfield M (2005) Veterinary epidemiology, 2nd edn. Blackwell Science, Oxford ((s 183))
Patridge EF, Bardyn TP (2018) Research Electronic Data Capture (REDCap). J Med Libr Assoc 106(1):142–144. https://doi.org/10.5195/jmla.2018.319
Oken M, Creech R, Tormey D et al (1982) Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 5:649–655
Klepstad P, Loge JH, Borchgrevink PC, Mendoza TR, Cleeland CS, Kaasa S (2002) The Norwegian brief pain inventory questionnaire: translation and validation in cancer pain patients. J Pain Symptom Manag 24:517–525
Scott NW, Fayers PM, Bottomley A, Aaronson NK, de Graeff A, Groenvold M, Koller M, Petersen MA, Sprangers MA, EORTC and the Quality of Life Cross-Cultural Meta-Analysis Group (2006) Comparing translations of the EORTC QLQ-C30 using differential item functioning analyses. Qual Life Res 15:1103–15
Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, Filiberti A, Flechtner H, Fleishman SB, de Haes JCJM, Kaasa S, Klee M, Osoba D, Razavi D, Rofe PB, Schraub S, Sneeuw K, Sullivan M, Takeda F, European Organization for Research and Treatment of Cancer Study Group on Quality of Life (1993) The European organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 85:365–76
Strömgren AS, Goldschmidt D, Groenvold M et al (2002) Self-assessment in cancer patients referred to palliative care: a study of feasibility and symptom epidemiology. Cancer 94(2):512–520. https://doi.org/10.1002/cncr.10222
Thronæs M, Raj SX, Brunelli C, Almberg SS, Vagnildhaug OM, Bruheim S, Helgheim B, Kaasa S, Knudsen AK (2016) Is it possible to detect an improvement in cancer pain management? A comparison of two Norwegian cross-sectional studies conducted 5 years apart. Support Care Cancer 24(6):2565–2574. https://doi.org/10.1007/s00520-015-3064-3
Caraceni A, Hanks GW, Kaasa S, Bennett MI, Brunelli C, Cherny N, Dale O, De Conno F, Fallon M, Hanna M, Haugen DF, Juhl G, King S, Klepstad P, Laugsand EA, Maltoni M, Mercadante S, Nabal M, Pigni A, Radbruch L, Reid C, Sjøgren P, Stone PC, Tassinari D, Zeppetella G, for the European Palliative Care Research Collaborative (EPCRC); on behalf of the European Association for Palliative Care (EAPC) (2012) Use of opioid analgesics in the treatment of cancer pain: evidence-based recommendations from the EAPC. Lancet Oncol 13:e58–e68
WHO (2018) Guidelines for the Pharmacological and Radiotherapeutic Management of Cancer Pain in Adults and Adolescents. World Health Organization, Geneva
van den Beuken-van Everdingen MH, Hochstenbach LM, Joosten EA, Tjan-Heijnen VC, Janssen DJ (2016 Jun) Update on prevalence of pain in patients with cancer: systematic review and meta-analysis. J Pain Symptom Manag 51(6):1070-1090.e9. https://doi.org/10.1016/j.jpainsymman.2015.12.340
Shkodra M, Brunelli C, Zecca E, Formaglio F, Bracchi P, Lo Dico S, Caputo M, Kaasa S, Caraceni A (2021) Neuropathic pain: clinical classification and assessment in patients with pain due to cancer. Pain 162(3):866–874
Finnerup NB, Haroutounian S, Kamerman P, Baron R, Bennett DL, Bouhassira D, Cruccu G, Freeman R, Hansson P, Nurmikko T et al (2016) Neuropathic pain: an updated grading system for research and clinical practice. Pain 157:1599–1606. https://doi.org/10.1097/j.pain.0000000000000492
Edwards HL, Mulvey MR, Bennett MI (2019) Cancer-related neuropathic pain. Cancers (Basel) 11(3):373
Roberto A, Deandrea S, Greco MT et al (2016) Prevalence of neuropathic pain in cancer patients: pooled estimates from a systematic review of published literature and results from a survey conducted in 50 Italian Palliative Care Centers. J Pain Symptom Manag 51(6):1091-1102.e4. https://doi.org/10.1016/j.jpainsymman.2015.12.336
Funding
This study was supported by a Grant from Oncological Research Foundation, Rigshospitalet, total 5000 DKK, for statistical analysis.
Author information
Authors and Affiliations
Contributions
Jonas Sørensen, Per Sjøgren and Geana Paula Kurita were involved in the conception and design of the study. Jonas Sørensen, Per Sjøgren and Geana Paula Kurita made the analysis plan. Jonas Sørensen, Stine Novrup Clemmensen, Tanja Vibeke Sørensen, and Katja Heinecke collected data. Jonas Sørensen and Geana Paula Kurita made the data analysis. Jonas Sørensen, Per Sjøgren, Stine Novrup Clemmensen, Tanja Vibeke Sørensen, Katja Heinecke and Geana Paula Kurita were involved in the interpretation of the data. Jonas Sørensen, Per Sjøgren and Geana Paula Kurita made the draft manuscript, which was revised and approved by all co-authors.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Collecting data from consenting inpatients was approved by the Danish Data Protection Agency (VD-2019–68). Non-interventional studies do not require approval from Ethic Committee in Denmark. All patients received oral and written information, and informed consent was obtained from all patients prior to inclusion.
Consent for publication
N/A
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key message
This study provides information on development of symptoms and treatment of adult cancer inpatients eight years after the establishment of a specialized palliative care (SPC) service in a comprehensive cancer center. The prevalence of symptoms during the period remained high; however, significant improvement on pain management and relief was found.
Rights and permissions
About this article
Cite this article
Sørensen, J., Sjøgren, P., Clemmensen, S.N. et al. Improvement of pain management in a comprehensive cancer center: a comparison of two cross-sectional studies 8 years apart. Support Care Cancer 30, 2037–2045 (2022). https://doi.org/10.1007/s00520-021-06614-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-021-06614-x



