Abstract
Purpose
Endometrial cancer is strongly linked to obesity and inactivity; however, increased physical activity has important benefits even in the absence of weight loss. Resistance (strength) training can deliver these benefits; yet few women participate in resistance exercise. The purpose of this study was to describe both physiological and functional changes following a home-based strength training intervention.
Methods
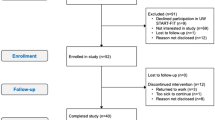
Forty post-treatment endometrial cancer survivors within 5 years of diagnosis were enrolled in a pilot randomized trial, comparing twice-weekly home-based strength exercise to wait list control. Participants conducted the exercises twice per week for 10 supervised weeks with 5 weeks of follow-up. Measures included DXA-measured lean mass, functional fitness assessments, blood biomarkers, and quality of life outcomes.
Results
On average, participants were 60.9 years old (SD = 8.7) with BMI of 39.9 kg/m2 (SD = 15.2). At baseline, participants had 51.2% (SD = 6.0) body fat, which was not different between groups. Improvements were seen in the 30-s chair sit to stand (d = .99), the 30-s arm curl (d = .91), and the 8-ft up-and-go test (d = .63). No changes were measured for HbA1c or C-reactive protein. No changes were observed for flexibility (chair sit and reach, back scratch tests), 6-min walk test, maximum handgrip test, anxiety, depression, fatigue, or self-efficacy for exercise.
Conclusions
Home-based muscle-strengthening exercise led to favorable and clinically relevant improvements in 3 of 7 physical function assessments. Physical function, body composition, blood biomarkers, and patient-reported outcomes were feasible to measure. These fitness improvements were observed over a relatively short time frame of 10 weeks.
Similar content being viewed by others
Data availability
Due to the nature of this research, participants of this study did not agree for their data to be shared publicly, so supporting data is not available.
Code availability
N/A
References
Sheikh MA et al (2014) USA endometrial cancer projections to 2030: should we be concerned? Future Oncol 10(16):2561–2568
Passarello K, Kurian S, Villanueva V (2019) Endometrial cancer: an overview of pathophysiology, management, and care. Semin Oncol Nurs 35(2):157–165. https://doi.org/10.1016/j.soncn.2019.02.002
Koutoukidis DA et al (2017) Recruitment, adherence, and retention of endometrial cancer survivors in a behavioural lifestyle programme: the Diet and Exercise in Uterine Cancer Survivors (DEUS) parallel randomised pilot trial. BMJ Open 7(10):e018015
Shisler R et al (2018) Life after endometrial cancer: a systematic review of patient-reported outcomes. Gynecol Oncol 148(2):403–413
Zhang X, Brown JC, Schmitz KH (2016) Association between body mass index and physical function among endometrial cancer survivors. PloS One 11(8):e0160954
Piercy KL et al (2018) The physical activity guidelines for Americans. JAMA 320(19):2020–2028
Campbell KL et al (2019) Exercise guidelines for cancer survivors: consensus statement from international multidisciplinary roundtable. Med Sci Sports Exerc 51(11):2375–2390
Arem H et al (2013) Prediagnosis body mass index, physical activity, and mortality in endometrial cancer patients. J Natl Cancer Inst 105(5):342–349
Crawford JJ et al (2016) A new paradigm for examining the correlates of aerobic, strength, and combined exercise: an application to gynecologic cancer survivors. Support Care Cancer 24(8):3533–3541
Riebe D et al (2018) ACSM’s guidelines for exercise testing and prescription. Wolters Kluwer. https://doi.org/10.1249/JSR.0b013e31829a68cf
Fader AN et al (2009) Endometrial cancer and obesity: epidemiology, biomarkers, prevention and survivorship. Gynecol Oncol 114(1):121–127
Anzuini F, Battistella A, Izzotti A (2011) Physical activity and cancer prevention: a review of current evidence and biological mechanisms. J Prev Med Hyg 52(4):174–180
Pollock ML et al (2000) AHA Science Advisory. Resistance exercise in individuals with and without cardiovascular disease: benefits, rationale, safety, and prescription: an advisory from the Committee on Exercise, Rehabilitation, and Prevention, Council on Clinical Cardiology, American Heart Association; Position paper endorsed by the American College of Sports Medicine. Circulation 101(7):828–833
Andersson EA et al (2017) Improving strength, power, muscle aerobic capacity, and glucose tolerance through short-term progressive strength training among elderly people. J Vis Exp (125). https://doi.org/10.3791/55518
McCarroll ML et al (2014) Self-efficacy, quality of life, and weight loss in overweight/obese endometrial cancer survivors (SUCCEED): a randomized controlled trial. Gynecol Oncol 132(2):397–402
Nock NL et al (2014) Rationale and design of REWARD (revving-up exercise for sustained weight loss by altering neurological reward and drive): a randomized trial in obese endometrial cancer survivors. Contemp Clin Trials 39(2):236–245
Kuroki LM et al (2015) Pre-operative assessment of muscle mass to predict surgical complications and prognosis in patients with endometrial cancer. Ann Surg Oncol 22(3):972–979
Thomas S, Reading J, Shephard RJ (1992) Revision of the Physical Activity Readiness Questionnaire (PAR-Q). Can J Sport Sci 17(4):338–345
Rock CL et al (2012) Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin 62(4):242–274
Powell KE et al (2018) The scientific foundation for the physical activity guidelines for Americans, 2nd edition. J Phys Act Health 1–11. https://doi.org/10.1123/jpah.2018-0618
Plotnikoff RC et al (2010) Multicomponent, home-based resistance training for obese adults with type 2 diabetes: a randomized controlled trial. Int J Obes (Lond) 34(12):1733–1741
Gorzelitz J et al (2021) Feasibility and acceptability of home-based strength training in endometrial cancer survivors. J Cancer Surviv. https://doi.org/10.1007/s11764-021-00990-3
Rikli RE, Jones CJ (2013) Development and validation of criterion-referenced clinically relevant fitness standards for maintaining physical independence in later years. Gerontologist 53(2):255–267
Kapur S, Kapur S, Zava D (2008) Cardiometabolic risk factors assessed by a finger stick dried blood spot method. J Diabetes Sci Technol 2(2):236–241
Brindle E et al (2010) Serum, plasma, and dried blood spot high-sensitivity C-reactive protein enzyme immunoassay for population research. J Immunol Methods 362(1–2):112–120
Harris PA et al (2019) The REDCap consortium: building an international community of software platform partners. J Biomed Inform 95:103208
Harris PA et al (2009) Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 42(2):377–381
Cella DF et al (1993) The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol 11(3):570–579
Resnick B, Jenkins LS (2000) Testing the reliability and validity of the Self-Efficacy for Exercise scale. Nurs Res 49(3):154–159
Cella D et al (2010) The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J Clin Epidemiol 63(11):1179–1194
Schalet BD et al (2016) Clinical validity of PROMIS depression, anxiety, and anger across diverse clinical samples. J Clin Epidemiol 73:119–127
Cook KF et al (2016) PROMIS measures of pain, fatigue, negative affect, physical function, and social function demonstrated clinical validity across a range of chronic conditions. J Clin Epidemiol 73:89–102
Campbell A et al (2005) A pilot study of a supervised group exercise programme as a rehabilitation treatment for women with breast cancer receiving adjuvant treatment. Eur J Oncol Nurs 9(1):56–63
Koutoukidis DA et al (2016) Diet and exercise in uterine cancer survivors (DEUS pilot) - piloting a healthy eating and physical activity program: study protocol for a randomized controlled trial. Trials 17(1):130
Arain M et al (2010) What is a pilot or feasibility study? A review of current practice and editorial policy. BMC Med Res Methodol 10:67
Rothman KJ (1990) No adjustments are needed for multiple comparisons. Epidemiology 1(1):43–46
Focht BC et al (2013) Resistance exercise interventions during and following cancer treatment: a systematic review. J Support Oncol 11(2):45–60
Beauchet O et al (2011) Timed up and go test and risk of falls in older adults: a systematic review. J Nutr Health Aging 15(10):933–938
Brown J, Harhay M, Harhay M (2015) Physical function as a prognostic biomarker among cancer survivors. Br J Cancer 112(1):194–198
Kelly TL, Wilson KE, Heymsfield SB (2009) Dual energy X-ray absorptiometry body composition reference values from NHANES. PloS One 4(9):e7038
Orsatti FL et al (2008) Plasma hormones, muscle mass and strength in resistance-trained postmenopausal women. Maturitas 59(4):394–404
Papatla K, Huang M, Slomovitz B (2016) The obese endometrial cancer patient: how do we effectively improve morbidity and mortality in this patient population? Ann Oncol 27(11):1988–1994
Strasser B et al (2013) Impact of resistance training in cancer survivors: a meta-analysis. Med Sci Sports Exerc 45(11):2080–2090
Schwartz CE et al (2006) The clinical significance of adaptation to changing health: a meta-analysis of response shift. Qual Life Res 15(9):1533–1550
Servaes P, Verhagen C, Bleijenberg G (2002) Fatigue in cancer patients during and after treatment: prevalence, correlates and interventions. Eur J Cancer 38(1):27–43
Acknowledgements
The authors would like to thank all the participants from this study, without which this work would not have been possible. The project was supported by the Clinical and Translational Science Award (CTSA) program, through the NIH National Center for Advancing Translational Sciences (NCATS), grant UL1TR002373.
Funding
PI (Bailey) UW Comprehensive Cancer Center Support (Sponsor DHHS, PHS, NIH)—2P30CA014520-44.
PI (Sugden Institutional Research Grant IRG) (Sponsor American Cancer Society Inc.)—IRG-15–213-51.
PI (Cadmus-Bertram) Feasibility Trial of Home-based Strength Training in Endometrial Cancer Survivors (Sponsor DHHS, PHS, NIH)—1R03CA230965-01A1.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Jessica Gorzelitz, supervised directly by Lisa Cadmus-Bertram. The first draft of the manuscript was written by Jessica Gorzelitz and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This study was approved by the University of Wisconsin-Madison’s Health Sciences Institutional Review Board (Protocol #2018–0953) and by the Carbone Comprehensive Cancer Center’s Protocol Review and Monitoring Committee (Protocol UW18013). Prior to enrollment of the first participant, the study was registered at clinicaltrials.gov (NCT03722030).
Consent to participate
Written informed consent was obtained from all individual participants included in the study.
Consent for publication
N/A
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gorzelitz, J.S., Stoller, S., Costanzo, E. et al. Improvements in strength and agility measures of functional fitness following a telehealth-delivered home-based exercise intervention in endometrial cancer survivors. Support Care Cancer 30, 447–455 (2022). https://doi.org/10.1007/s00520-021-06415-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-021-06415-2