Abstract
Purpose
Patients with preexisting autoimmune disease (PAD) are often excluded from clinical trials assessing immune checkpoint inhibitors (ICIs). Therefore, the safety of ICI therapy in patients with PAD remains unclear. Herein, we evaluated the incidence of immune-related adverse events (irAEs) in patients with PAD when compared with non-PAD patients.
Methods
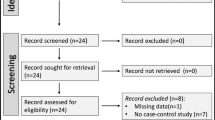
We searched MEDLINE/PubMed, Web of Science, and Google Scholar for eligible studies from inception to January 2021. Observational studies reporting the incidence of irAEs in patients with and without PAD were included. We then performed a meta-analysis of eligible studies using forest plots. The primary endpoint of this study was the incidence rate of irAEs between patients with and without PAD.
Results
We identified three prospective and three retrospective studies involving 206 patients with PAD and 3078 patients without PAD. In the meta-analysis, 128 patients with PAD (62.1%) experienced irAEs, which occurred in 51.9% of non-PAD patients, resulting in an odds ratio (OR) of 2.14 (95% confidence interval [CI] 1.58–2.89). In the subgroup analysis, the incidence of irAEs was significantly higher in patients with PAD (OR = 2.19, 95% CI [1.55–3.08]). Furthermore, no significant heterogeneity or publication bias was detected, indicating that our meta-analysis could be generalized to clinical settings.
Conclusion
This meta-analysis demonstrated that PAD was a risk factor for irAE incidence. These results suggest that monitoring the occurrence of irAEs in patients with PAD is required to manage irAEs appropriately.




Similar content being viewed by others
Data availability
The dataset used for this study are available from the corresponding author on reasonable request.
References
Dine J, Gordon R, Shames Y, Kasler MK, Barton-Burke M (2017) Immune checkpoint inhibitors: An innovation in immunotherapy for the treatment and management of patients with cancer. Asia Pac J Oncol Nurs 4:127–135
Postow MA, Sidlow R, Hellmann MD (2018) Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med 378:158–168
Blidner AG, Choi J, Cooksley T et al (2020) Cancer immunotherapy-related adverse events: causes and challenges. Support Care Cancer 28:6111–6117
Brahmer JR, Lacchetti C, Schneider BJ et al (2018) Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 36:1714–1768
Rapoport BL, Anderson R, Cooksley T, Johnson DB (2020) MASCC 2020 recommendations for the management of immune-related adverse events of patients undergoing treatment with immune checkpoint inhibitors. Support Care Cancer 28:6107–6110
Johnson DB, Sullivan RJ, Menzies AM (2017) Immune checkpoint inhibitors in challenging populations. Cancer 123:1904–1911
Xie W, Huang H, Xiao S et al (2020) Immune checkpoint inhibitors therapies in patients with cancer and preexisting autoimmune diseases: A meta-analysis of observational studies. Autoimmun Rev 19:102687
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group (2009) Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA statement. BMJ 339:b2535
Begg CB, Mazumdar M (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50:1088–1101
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315:629–634
Bair SM, Strelec LE, Feldman TA et al (2019) Outcomes and toxicities of programmed Death-1 (PD-1) inhibitors in Hodgkin lymphoma patients in the United States: a real-world, multicenter retrospective analysis. Oncologist 24:955–962
Cortellini A, Buti S, Santini D et al (2019) Clinical outcomes of patients with advanced cancer and preexisting autoimmune diseases treated with anti-programmed Death-1 immunotherapy: a real-world transverse study. Oncologist 24:e327–e337
Kartolo A, Sattar J, Sahai V, Baetz T, Lakoff JM (2018) Predictors of immunotherapy-induced immune-related adverse events. Curr Oncol 25:e403–e410
Danlos FX, Voisin AL, Dyevre V et al (2018) Safety and efficacy of anti-programmed death 1 antibodies in patients with cancer and preexisting autoimmune or inflammatory disease. Eur J Cancer 91:21–29
Loriot Y, Sternberg CN, Castellano D et al (2020) Safety and efficacy of atezolizumab in patients with autoimmune disease: subgroup analysis of the SAUL study in locally advanced/metastatic urinary tract carcinoma. Eur J Cancer 138:202–211
Schadendorf D, Ascierto PA, Haanen J et al (2019) Safety and efficacy of nivolumab in challenging subgroups with advanced melanoma who progressed on or after ipilimumab treatment: a single-arm, open-label, phase II study (CheckMate 172). Eur J Cancer 121:144–153
Cooksley T, Girotra M, Ginex P et al (2020) Multinational Association of Supportive Care in Cancer (MASCC) 2020 clinical practice recommendations for the management of immune checkpoint inhibitor endocrinopathies and the role of advanced practice providers in the management of immune-mediated toxicities. Support Care Cancer 28:6175–6181
Bender DA, Heilbroner SP, Wang TJC et al (2020) Increased rates of immunosuppressive treatment and hospitalization after checkpoint inhibitor therapy in cancer patients with autoimmune disease. J Immunother Cancer 8:e001627
Kehl KL, Yang S, Awad MM et al (2019) Preexisting autoimmune disease and the risk of immune-related adverse events among patients receiving checkpoint inhibitors for cancer. Cancer Immunol Immunother 68:917–926
El Majzoub I, Qdaisat A, Thein KZ et al (2019) Adverse effects of immune checkpoint therapy in cancer patients visiting the emergency department of a comprehensive cancer center. Ann Emerg Med 73:79–87
Cooksley T, Gupta A, Al-Sayed T, Lorigan P (2020) Emergency presentations in patients treated with immune checkpoint inhibitors. Eur J Cancer 130:193–197
Toi Y, Sugawara S, Sugisaka J et al (2019) Profiling preexisting antibodies in patients treated with anti-PD-1 therapy for advanced non-small cell lung cancer. JAMA Oncol 5:376–383
Sakakida T, Ishikawa T, Chihara Y et al (2020) Safety and efficacy of PD-1/PD-L1 blockade in patients with preexisting antinuclear antibodies. Clin Transl Oncol 22:919–927
Abdel-Wahab N, Shah M, Lopez-Olivo MA, Suarez-Almazor ME (2018) Use of immune checkpoint inhibitors in the treatment of patients with cancer and preexisting autoimmune disease: a systematic review. Ann Intern Med 168:121–130
Wahren-Herlenius M, Dörner T (2013) Immunopathogenic mechanisms of systemic autoimmune disease. Lancet 382:819–831
Khan S, Gerber DE (2020) Autoimmunity, checkpoint inhibitor therapy and immune-related adverse events: A review. Semin Cancer Biol 64:93–101
Vavricka SR, Schoepfer A, Scharl M et al (2015) Extraintestinal manifestations of inflammatory bowel disease. Inflam Bowel Dis 21:1982–1992
Spagnolo P, Lee JS, Sverzellati N, Rossi G, Cottin V (2018) The lung in rheumatoid arthritis: Focus on interstitial lung disease. Arthritis Rheumatol 70:1544–1554
Arbour KC, Mezquita L, Long N et al (2018) Impact of baseline steroids on efficacy of programmed cell Death-1 and programmed death-ligand 1 blockade in patients with non-small-cell lung cancer. J Clin Oncol 36:2872–2878
Akamatsu H, Murakami E, Oyanagi J et al (2020) Immune-related adverse events by immune checkpoint inhibitors significantly predict durable efficacy even in responders with advanced non-small cell lung cancer. Oncologist 25:e679–e683
Author information
Authors and Affiliations
Contributions
AY, YS, and MK designed the study. AY and MK conducted the study and analyzed the data. AY, YS, MK, KO, KN, AF, YT, and MS contributed to the writing of the manuscript. All authors reviewed the results and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix: search strategy
Appendix: search strategy
We searched the related records in three databases (MEDLINE/PubMed, Web of Science, and Google Scholar), with no language restriction, from database inception to January 31, 2021. In the MEDLINE/PubMed database, we used PubMed Advanced Search Builder with the following terms: “neoplasm” AND “immune checkpoint inhibitors” AND “autoimmune disease” in all fields. In the Web of Science database, we selected all databases and conducted a basic search with the following terms: “neoplasm” AND “immune checkpoint inhibitors” AND “autoimmune disease” in the topic field. In Google Scholar, we searched articles using the advanced search feature and with the following terms: “neoplasm” AND “immune checkpoint inhibitors” AND “autoimmune disease,” with all of the words appearing anywhere in the article. Default values were chosen for all other settings.
Rights and permissions
About this article
Cite this article
Yamaguchi, A., Saito, Y., Okamoto, K. et al. Preexisting autoimmune disease is a risk factor for immune-related adverse events: a meta-analysis. Support Care Cancer 29, 7747–7753 (2021). https://doi.org/10.1007/s00520-021-06359-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-021-06359-7