Abstract
Objective
To describe overall survival (OS) in 90 days and to evaluate the prognostic factors in patients with advanced cancer and COVID-19.
Methods
This is a retrospective cohort study carried out at the Palliative Care Unit of the Brazilian National Cancer Institute. Patients with advanced cancer and COVID-19 confirmed by Reverse Transcription Polymerase Chain Reaction were included. Kaplan-Meier’s curves, log-rank test, and Cox regression were performed.
Results
Eighty-three inpatients were selected. The average age was 61.4 (±12.6) years, with a higher proportion of women (73.4%). The most prevalent tumor type was breast (36.7%), followed by gastrointestinal tract (20.3%). The OS was 32 [interquartile range (IQR): 6–70] days, and at the end of the follow-up period, 17 patients (20.5%) were alive and 66 (79.5%) had died. Patients with advanced cancer and COVID-19 and who were 60–74 years old [hazard ratio (HR): 2.03; 95% confidence interval (CI): 1.09–3.78], with lung tumors (HR: 17.50; 95% CI: 1.70–28.34), with lung metastasis (HR: 4.21; 95% CI: 2.17–8.15), and with chronic obstructive pulmonary disease (HR: 4.92; 95% CI: 1.01–24.69) had higher risk of death in 90 days.
Conclusion
The age of 60–74 years old, lung tumors (primary or metastases), and the presence of chronic obstructive pulmonary disease were considered independent prognostic factors in patients with advanced cancer and COVID-19.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Patients with cancer are probably more susceptible to infections such as the new coronavirus that causes the coronavirus disease 2019 (COVID-19) [1]. Moreover, they present higher mortality that can be explained by an immunocompromised status, due to the cancer itself, surgery or antineoplastic therapy, chronic use of steroids, and others factors [2,3,4]. In this context, patients with advanced cancer in palliative care typically have a reduced overall survival (OS) [5] and those suffering from COVID-19 disease will probably experience an even lower OS.
Information on the impact of COVID-19 infection on the OS of patients with cancer, as well as the associated prognostic factors, is still scarce. By analyzing data of the Brazilian cohort, Melo et al. [6] showed that the rates of complications and specific death of patients with cancer infected by the virus were high. In a meta-analysis study carried out by Desai et al. [7], with data from 2,922 patients with cancer, of different stages, and who were hospitalized with COVID-19, it was found that males, together with an interaction between the patient’s median age and recent active cancer therapy, were factors related to the mortality.
Determining prognosis in advanced cancer is of key importance to guide the establishment of the care plan [8]. In context of end-of-life care, for example, probably, one of the factors of greatest suffering is the absence of family members and the loved ones, since infection by the COVID-19 demands isolation from the patient. With a better understanding of the factors that can predict worse outcomes, the team has the possibility of offering a better support to the family and patients in preparing for death using new ways for humanizing the death process, such as the use of information technologies, although it seems contradictory, contributing to the promotion of communication at a distance between hospitalized patients and their family members and the loved ones. However, although there are different recognized prognostic factors in advanced disease [8], there is still no concrete evidence about its clinical utility in the context of COVID-19.
Thinking about that, the objective of the present study was (i) to describe OS in 90 days, and (ii) to evaluate the prognostic factors in patients with advanced cancer admitted to the Palliative Care Unit (PCU) of the Brazilian National Cancer Institute (INCA), with COVID-19.
Methods
This is a clinical, observational, retrospective cohort study, with data extracted from the medical records of all inpatients with advanced cancer admitted to the exclusive PCU at the INCA, in the city of Rio de Janeiro/RJ, during the period from March 19 to July, 2020, with COVID-19. The study was approved by the INCA Ethics Committee (CAAE: 31053220.0.0000.5274) that exempted the need of individual consent for being a retrospective observational study and for considering only the use of information provided in medical records available at the institution.
INCA is an agency under the Ministry of Health and is the largest public institution of reference for the development and coordination of integrated cancer prevention and control actions in Brazil. The PCU receives patients referred from all other care units of INCA after their treatment possibilities have finished and cure has not been achieved, after the disease has progressed while under treatment, and the patients do not benefit anymore from its continuity or have the clinical condition worsened, and the continuity of specific therapeutic activities aimed at cytoreduction (be it the chemotherapy lines, surgical interventions, radiotherapy, and radiosurgery). At the PCU, the goal of treatment is to control symptoms and promote quality of life and death. The patients had generalized malignant disease or advanced local tumor growth and were not receiving any antineoplastic treatment with control intent. Approximately 1500 patients were admitted at the PCU in 2020.
Patients were identified through the Monitoring System of the Hospital Infection Control Commission. From there, hospitalized patients of both genders, aged > 20 years, with a confirmed diagnosis of locally advanced (E III) or distant metastatic progression (E IV), regardless of tumor location (proven by histological, cytological, or radiological evidence), and COVID-19 confirmed by reverse transcription polymerase chain reaction (RT-PCR) [9, 10] were selected.
Data collection
Regarding the moment of the patient’s admission, the following were collected: demographic data [age (< 60 vs. 60–74 vs. > 74 years old) and gender (male vs. female)], clinical characteristics [primary tumor site (breast vs. gastrointestinal tract vs. head and neck vs. urological vs. gynecological vs. connective bone tissue vs. lung vs. other), metastases (local vs. distant), lung metastasis (yes vs. no), admission less than 60 days since the last surgery (yes vs. no), chemotherapy (yes vs. no) and radiation therapy (yes vs. no), comorbidities (diabetes mellitus: yes vs. no; systemic arterial hypertension: yes vs. no; cardiovascular disease: yes vs. no; chronic obstructive pulmonary disease: yes vs. no)], and laboratory tests categorized according to distribution tertiles [hemoglobin (1st vs. 2nd and 3rd tertiles), leukocytes (3rd vs. 1st and 2nd tertiles), albumin (1st vs. 2nd and 3rd tertiles), and C-reactive protein (3rd vs. 1st and 2nd tertiles)].
In addition, medications used during hospitalization (antibiotic: yes vs. no; corticosteroids: yes vs. no; and anticoagulant: yes vs. no) were collected.
OS was defined as the time interval, in days, between the date of beginning of palliative care and the date of death from any cause. All patients who remained alive after the end of the period of study (follow-up period: 90 days) were censured.
Statistical analysis
The analysis was performed using the STATA 13.1. Kaplan-Meier’s method and the log-rank test were used to compare OS according to the variables. The Cox proportional hazards models were used to assess hazard ratios (HRs). To reduce the chance of excluding important variables from the model, all factors with a p-value < 0.20 in the univariate were included in the multivariate analysis. The final model was obtained through the stepwise backward procedure and it included all variables with p-value < 0.05.
Results
Eighty-three inpatients with advanced cancer and COVID-19 were selected. The average age was 61.4 (± 12.6) years old, with a higher proportion of women (73.4%), primary tumor site in breast (36.7%), and gastrointestinal tract (20.3%). Patients with distant metastasis accounted for more than half of the cases (n = 71; 89.9%) and 32 (40.5%) had lung metastasis (Table 1).
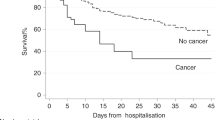
At the end of the follow-up period of 90 days, 17 patients (20.5%) (average age was 64.1 (± 10.2) years old) were alive and 66 (79.5%) (average age was 60.6 (± 10.4) years old) had died. The OS was 32 (interquartile range (IQR): 6–70) days (data not shown). Lower OS was verified among patients 60–74 years old (11 (IQR: 6–35) days; p-value 0.029), with lung metastasis (8 (IQR: 2–37) days; p-value 0.005), who had received radiotherapy in < 60 days (8 (IQR: 8–26) days; p-value 0.015), and who used therapeutic anticoagulation (12 (IQR: 5–42) days; p-value 0.024) (Table 2). These variables with a different probability of survival between the groups are shown graphically in Fig. 1.
According to the multivariate analysis, 60–74 years old (HR: 2.03; 95% confidence interval (CI): 1.09–3.78), lung tumors (HR: 17.50; 95% CI: 1.70–28.34), lung metastasis (HR: 4.21; 95% CI: 2.17–8.15), and chronic obstructive pulmonary disease (HR: 4.92; 95% CI: 1.01–24.69) were independent prognostic factors for OS in patients with advanced cancer and COVID-19 (Table 3).
Discussion
This work was developed in a national reference cancer center and had two main results. First, it verified that patients in palliative care with advanced cancer and COVID-19 had a restricted OS. Second, it demonstrated that the age of 60–74 years and the presence of lung tumors (primary site or metastasis) and chronic obstructive pulmonary disease predict OS were considered independent prognostic factors in this group.
Palliative care aims to improve quality of life and death to those with a life-threatening disease. During an epidemic, the palliative care services must respond briefly seeking new ways of working to maintain these goals [11, 12]. Improving prognosis can contribute to this. It is also important to highlight that Brazil was classified as one of the worst countries in relation to the growth of the spread of COVID-19 and in relation to the lethality rate of the disease. Rio de Janeiro is the city with most deaths from COVID-19 in the country, reaching in February 2021 17,535 deaths. These results are relevant and can assist us in developing a strategy to better meet the demands of patients in these conditions.
Patients in palliative care with advanced cancer and COVID-19 had a median OS of 32 (IQR: 6–70) days. Although the scientific literature still lacks data about the OS of this group, a previous study has reported that patients with advanced cancer, seen at the same center of treatment, but without infection by the virus, had a median OS of 53 (IQR: 20–90) days [5]. Silva et al. [13], in an observational prospective cohort study, conducted at the same center of treatment before the pandemic, showed that patients with advanced cancer had a median OS of 39 (IQR: 26–90) days. It should be noted that these two studies [5, 13] were developed with advanced cancer inpatients and outpatients who had their first consultation at the PCU. The mean age was 62 years [5, 13], the majority of patients were female (56.4% [5] and 57.1% [13]) and had gastrointestinal tract tumor [5, 13] and metastatic distant disease (85.3% [5] and 80.4% [13]).
Patients who were 60 to 74 years old had a double risk of dying compared to younger ones. It is known that advancing age is associated with a greater likelihood of the occurrence of chronic diseases and a reduction in the response capacity of the immune system [14]. Liu et al. [15], through a meta-analysis, found that in six selected studies, the age > 65 years old was associated with an increased risk of death (relative risk (RR): 1.27; 95% CI: 1.08–1.49). According to Ruthrich et al. [16], advanced age was associated with higher death rates in a cohort of 435 patients with cancer and confirmed SARS-CoV-2 infection, and enrolled between March 16 and August 31, 2020.
Such an association was not found in those > 74 years old. One of the factors that can justify this result is the incident form of neoplasms in old age. The majority of these patients were female and had breast cancer. Thus, with our sample size, we have only 12 patients in this age group, which may have impaired the power of statistical analysis in this group. Larger sample studies need to be developed.
Previous pulmonary involvement disease, either by primary tumor site or metastatic disease, as well as chronic obstructive pulmonary disease, was a predictor of death in this group. As verified by Dai et al. [17], patients with lung cancer had the second-highest risk levels, with death rates at 18.2%. Melo et al. [6] identified the presence of lung metastasis as a risk factor for death (odds ratio (OR): 8.6; 95% CI: 3.5–21.3), and Liu et al. [15] found the same association in relation to the presence of chronic obstructive pulmonary disease (RR: 1.47; 95% CI: 1.09–1.98).
Knowing that advanced cancer patients with COVID-19 with age between 60 and 74 years old and previous pulmonary involvement disease have a higher risk of death within 90 days can help to improve the palliative care offer. Patients with these characteristics need even greater care to prevent contamination by the virus, including greater monitoring by call center and home assistance to reduce their circulation in environments common to other individuals and patients.
Although we did not find any prognostic association related to sex, it is known that severe cases of COVID-19 and mortality have been associated with male patients in different studies [16, 18, 19]. Two factors can explain our data: First, the high prevalence of female patients in the general scope of the population often referred to the PCU, as reported by studies carried out in the same place [5, 13, 20], and second, the high proportion of patients with breast tumors, a disease with a predominant female characteristic, being considered the most prevalent in this sample. All of this may have influenced the results and be responsible for some bias in this regard.
Therefore, among the limitations of this study, it is important to highlight the high frequency of female patients with advanced cancer and COVID-19 in this study, which may have an impact on the generalization of results. In addition, the study was carried out in a single institution, with a retrospective design and had a restricted sample size. Multicenter studies with prospective design should be performed and could confirm the predictive value of these parameters.
In conclusion, our results demonstrate that the age of 60–74 years, lung tumors (primary or metastases), and chronic obstructive pulmonary disease were independent prognostic factors. More studies should be developed to better clarify these aspects.
Data availability
Data are available only in the research group’s database.
Code availability
NA.
References
Tian Y, Qiu C, Wang C et al (2021) Cancer associates with risk and severe events of COVID-19: a systematic review and meta-analysis. Int J Cancer 148(2):363–374
Dolan RD, McSorley ST, Horgan PG et al (2017) The role of the systemic inflammatory response in predicting outcomes in patients with advanced inoperable cancer: systematic review and meta-analysis. Crit Rev Oncol Hematol 116:134–146
Gray S, Axelsson B (2018) The prevalence of deranged C-reactive protein and albumin in patients with incurable cancer approaching death. PLoS One 13(3):e0193693
Shalapour S, Karin M (2015) Immunity, inflammation, and cancer: an eternal fight between good and evil. J Clin Invest 125:3347–3355
Wiegert EVM, Oliveira LC, Calixto-Lima L et al (2020) Cancer cachexia: comparing diagnostic criteria in patients with incurable cancer. Nutrition 79-80:110945
Melo AC, Thuler LCS, da Silva JL et al (2020) Cancer inpatients with COVID-19: a report from the Brazilian National Cancer Institute. PLoS One 15(10):e0241261
Desai A, Gupta R, Advani S, et al (2020) Mortality in hospitalized patients with cancer and coronavirus disease 2019: a systematic review and meta-analysis of cohort studies. Cancer:1-10
Hui D, Paiva CE, Del Fabbro EG et al (2019) Prognostication in advanced cancer: update and directions for future research. Support Care Cancer 27(6):1973–1984
Sampaio SGSM, Dias AM, Freitas R et al (2020). Evaluation of the criteria adopted to identify suspected cases of COVID-19 in the emergency department service of a referral palliative oncology care unit. Am J Hosp Palliat Care :1-5
World Health Organization (2020) Clinical management of severe acute respiratory infection when COVID-19 is suspected. Accessed Sep 3 2020. Available from: https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected
Etkind SN, Bone AE, Lovell N, Cripps RL, Harding R, Higginson IJ, Sleeman KE (2020) The role and response of palliative care and hospice services in epidemics and pandemics: a rapid review to inform practice during the COVID-19 pandemic. J Pain Symptom Manag 60(1):e31–e40
Freitas R, Oliveira L, Rosa K et al (2020) Cuidados Paliativos em Pacientes com Câncer Avançado e Covid-19. Rev Bras Cancerol 66:e-1077
Silva GA, Wiegert EVM, Calixto-Lima L et al (2020) Clinical utility of the modified Glasgow Prognostic Score to classify cachexia in patients with advanced cancer in palliative care. Clin Nutr 39(5):1587–1592
Aging M-JF, Sex M (2020) Obesity, and metabolic inflammation create the perfect storm for COVID-19. Diabetes 69(9):1857–1863
Liu Y, Lu H, Wang W et al (2020) Clinical risk factors for mortality in patients with cancer and COVID-19: a systematic review and meta-analysis of recent observational studies. Expert Rev Anticancer Ther 21:1–13
Rüthrich MM, Giessen-Jung C, Borgmann S et al (2021) COVID-19 in cancer patients: clinical characteristics and outcome—an analysis of the LEOSS registry. Ann Hematol 100:383–393
Dai M, Liu D, Liu M, Zhou F, Li G, Chen Z, Zhang Z, You H, Wu M, Zheng Q, Xiong Y, Xiong H, Wang C, Chen C, Xiong F, Zhang Y, Peng Y, Ge S, Zhen B, Yu T, Wang L, Wang H, Liu Y, Chen Y, Mei J, Gao X, Li Z, Gan L, He C, Li Z, Shi Y, Qi Y, Yang J, Tenen DG, Chai L, Mucci LA, Santillana M, Cai H (2020) Patients with cancer appear more vulnerable to SARS-CoV-2: a multicenter study during the COVID-19 outbreak. Cancer Discov 10(6):783–791
Kuderer NM, Choueiri TK, Shah DP, Shyr Y, Rubinstein SM, Rivera DR, Shete S, Hsu CY, Desai A, de Lima Lopes G Jr, Grivas P, Painter CA, Peters S, Thompson MA, Bakouny Z, Batist G, Bekaii-Saab T, Bilen MA, Bouganim N, Larroya MB, Castellano D, del Prete SA, Doroshow DB, Egan PC, Elkrief A, Farmakiotis D, Flora D, Galsky MD, Glover MJ, Griffiths EA, Gulati AP, Gupta S, Hafez N, Halfdanarson TR, Hawley JE, Hsu E, Kasi A, Khaki AR, Lemmon CA, Lewis C, Logan B, Masters T, McKay RR, Mesa RA, Morgans AK, Mulcahy MF, Panagiotou OA, Peddi P, Pennell NA, Reynolds K, Rosen LR, Rosovsky R, Salazar M, Schmidt A, Shah SA, Shaya JA, Steinharter J, Stockerl-Goldstein KE, Subbiah S, Vinh DC, Wehbe FH, Weissmann LB, Wu JTY, Wulff-Burchfield E, Xie Z, Yeh A, Yu PP, Zhou AY, Zubiri L, Mishra S, Lyman GH, Rini BI, Warner JL, Abidi M, Acoba JD, Agarwal N, Ahmad S, Ajmera A, Altman J, Angevine AH, Azad N, Bar MH, Bardia A, Barnholtz-Sloan J, Barrow B, Bashir B, Belenkaya R, Berg S, Bernicker EH, Bestvina C, Bishnoi R, Boland G, Bonnen M, Bouchard G, Bowles DW, Busser F, Cabal A, Caimi P, Carducci T, Casulo C, Chen JL, Clement JM, Chism D, Cook E, Curran C, Daher A, Dailey M, Dahiya S, Deeken J, Demetri GD, DiLullo S, Duma N, Elias R, Faller B, Fecher LA, Feldman LE, Friese CR, Fu P, Fu J, Futreal A, Gainor J, Garcia J, Gill DM, Gillaspie EA, Giordano A, Glace (M)G, Grothey A, Gulati S, Gurley M, Halmos B, Herbst R, Hershman D, Hoskins K, Jain RK, Jabbour S, Jha A, Johnson DB, Joshi M, Kelleher K, Kharofa J, Khan H, Knoble J, Koshkin VS, Kulkarni AA, Lammers PE, Leighton JC Jr, Lewis MA, Li X, Li A, Lo KMS, Loaiza-Bonilla A, LoRusso P, Low CA, Lustberg MB, Mahadevan D, Mansoor AH, Marcum M, Markham MJ, Handy Marshall C, Mashru SH, Matar S, McNair C, McWeeney S, Mehnert JM, Menendez A, Menon H, Messmer M, Monahan R, Mushtaq S, Nagaraj G, Nagle S, Naidoo J, Nakayama JM, Narayan V, Nelson HH, Nemecek ER, Nguyen R, Nuzzo PV, Oberstein PE, Olszewski AJ, Owenby S, Pasquinelli MM, Philip J, Prabhakaran S, Puc M, Ramirez A, Rathmann J, Revankar SG, Rho YS, Rhodes TD, Rice RL, Riely GJ, Riess J, Rink C, Robilotti EV, Rosenstein L, Routy B, Rovito MA, Saif MW, Sanyal A, Schapira L, Schwartz C, Serrano O, Shah M, Shah C, Shaw G, Shergill A, Shouse G, Soares HP, Solorzano CC, Srivastava PK, Stauffer K, Stover DG, Stratton J, Stratton C, Subbiah V, Tamimi R, Tannir NM, Topaloglu U, van Allen E, van Loon S, Vega-Luna K, Venepalli N, Verma AK, Vikas P, Wall S, Weinstein PL, Weiss M, Wise-Draper T, Wood WA, Xu W(V), Yackzan S, Zacks R, Zhang T, Zimmer AJ, West J (2020) Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet 395(10241):1907–1918
Lee LY, Cazier JB, Angelis V, Arnold R, Bisht V, Campton NA, Chackathayil J, Cheng VW, Curley HM, Fittall MW, Freeman-Mills L, Gennatas S, Goel A, Hartley S, Hughes DJ, Kerr D, Lee AJ, Lee RJ, McGrath S, Middleton CP, Murugaesu N, Newsom-Davis T, Okines AF, Olsson-Brown AC, Palles C, Pan Y, Pettengell R, Powles T, Protheroe EA, Purshouse K, Sharma-Oates A, Sivakumar S, Smith AJ, Starkey T, Turnbull CD, Várnai C, Yousaf N, UK Coronavirus Monitoring Project Team, Kerr R, Middleton G (2020) COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet 395(10241):1919–1926
Oliveira LC, Abreu GT, Lima LC et al (2020) Quality of life and its relation with nutritional status in patients with incurable cancer in palliative care. Support Care Cancer 28(10):4971–4978
Acknowledgements
To the entire care work force of the unit that has dedicated itself very hard to the care of patients in a pandemic period of COVID-19. Special thanks to the great friend Nelson Santos (in memoriam).
Author information
Authors and Affiliations
Contributions
Oliviera LC, Rosa KSC, Borsatto AZ, Oliveira LAF, Freitas R, and Sampaio SGSM contributed to the conception and design of the research; Oliviera LC, Rosa KSC, and Borsatto AZ contributed to the acquisition and analysis of the data; Oliveira LAF, Freitas R, and Sampaio SGSM contributed to the interpretation of the data; and Oliviera LC, Rosa KSC, Borsatto AZ, Oliveira LAF, Freitas R, and Sampaio SGSM drafted the manuscript. All authors critically revised the manuscript, agree to be fully accountable for ensuring the integrity and accuracy of the work, and read and approved the final manuscript. All authors critically revised the manuscript, and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the INCA Ethics Committee (CAAE: 31053220.0.0000.5274). The study has exempted the need for individual consent, according to INCA Ethics Committee.
Consent for publication
All authors have seen and approved the contents of the submitted manuscript for publication.
Competing interest
The authors declare no competing interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
de Oliveira, L.C., da Costa Rosa, K.S., Borsatto, A.Z. et al. Prognostic factors in patients with advanced cancer and COVID-19: a cohort from the Palliative Care Unit of the Brazilian National Cancer Institute. Support Care Cancer 29, 6005–6012 (2021). https://doi.org/10.1007/s00520-021-06149-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-021-06149-1