Abstract
Background
Even with significant advances in surgical techniques and treatment, salvage chemotherapy remains the major treatment strategy for patients with unresectable or metastatic gastric cancer (GC). Practical and technical advances have simplified safe and convenient use of supplemental home parenteral nutrition (HPN). We aimed to clarify the role of HPN in patients with incurable GC undergoing salvage chemotherapy.
Methods
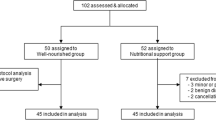
We enrolled 25 patients with GC with a nutritional risk index (NRI) of ≦ 97.5 undergoing HPN. Their nutritional status, laboratory data, and quality of life (QoL) were analyzed using the Research and Treatment of Cancer quality of life questionnaire-C30 before and after HPN administration at 0.5, 1, 2, and 3 months. We enrolled 25 patients with an NRI of > 97.5 not undergoing HPN as the control group.
Results
Total protein (P = 0.008), prealbumin (P < 0.001), and total cholesterol (P = 0.023) levels improved significantly after 0.5 months of HPN administration. The study group also demonstrated a marked improvement in nitrogen balance (P = 0.004) and prealbumin levels (P < 0.012) after 1 month. Gains in body weight after 1 month and body mass index after 2 months of HPN administration remained comparable with those of the control group. Global QoL scores were maintained and comparable with those of the control group.
Conclusions
Supplemental HPN therapy for malnourished patients with unresectable or metastatic GC undergoing salvage chemotherapy is feasible and revealed marked improvement in nutritional status. Early HPN intervention should be considered an important part of palliative treatment for advanced GC.

Similar content being viewed by others
Availability of data and materials
The data and materials analyzed in the current study are available from the corresponding author on reasonable requests.
References
Ministry of Health and Culture (2019) http://www.mohw.gov.tw
Andreyev HJ, Norman AR, Oates J, Cunningham D (1998) Why do patients with weight loss have a worse outcome when undergoing chemotherapy for gastrointestinal malignancies? Eur J Cancer 34(4):503–509
Ross PJ, Ashley S, Norton A, Priest K, Waters JS, Eisen T, Smith IE, O'Brien ME (2004) Do patients with weight loss have a worse outcome when undergoing chemotherapy for lung cancers? Br J Cancer 90(10):1905–1911. https://doi.org/10.1038/sj.bjc.6601781
Massicotte MH, Borget I, Broutin S, Baracos VE, Leboulleux S, Baudin E, Paci A, Deroussent A, Schlumberger M, Antoun S (2013) Body composition variation and impact of low skeletal muscle mass in patients with advanced medullary thyroid carcinoma treated with vandetanib: results from a placebo-controlled study. J Clin Endocrinol Metab 98(6):2401–2408. https://doi.org/10.1210/jc.2013-1115
Arends J, Bachmann P, Baracos V, Barthelemy N, Bertz H, Bozzetti F, Fearon K, Hutterer E, Isenring E, Kaasa S, Krznaric Z, Laird B, Larsson M, Laviano A, Muhlebach S, Muscaritoli M, Oldervoll L, Ravasco P, Solheim T, Strasser F, de van der Schueren M, Preiser JC (2017) ESPEN guidelines on nutrition in cancer patients. Clin Nutr 36(1):11–48. https://doi.org/10.1016/j.clnu.2016.07.015
Druml C, Ballmer PE, Druml W, Oehmichen F, Shenkin A, Singer P, Soeters P, Weimann A, Bischoff SC (2016) ESPEN guideline on ethical aspects of artificial nutrition and hydration. Clin Nutr 35(3):545–556. https://doi.org/10.1016/j.clnu.2016.02.006
Bozzetti F (2019) The role of parenteral nutrition in patients with malignant bowel obstruction. Support Care Cancer. https://doi.org/10.1007/s00520-019-04948-1
Altmann GG (1972) Influence of starvation and refeeding on mucosal size and epithelial renewal in the rat small intestine. Am J Anat 133(4):391–400. https://doi.org/10.1002/aja.1001330403
Roxburgh CS, McMillan DC (2014) Cancer and systemic inflammation: treat the tumour and treat the host. Br J Cancer 110(6):1409–1412. https://doi.org/10.1038/bjc.2014.90
Dantzer R (2004) Cytokine-induced sickness behaviour: a neuroimmune response to activation of innate immunity. Eur J Pharmacol 500(1-3):399–411. https://doi.org/10.1016/j.ejphar.2004.07.040
Prado CM, Baracos VE, McCargar LJ, Mourtzakis M, Mulder KE, Reiman T, Butts CA, Scarfe AG, Sawyer MB (2007) Body composition as an independent determinant of 5-fluorouracil-based chemotherapy toxicity. Clin Cancer Res 13(11):3264–3268. https://doi.org/10.1158/1078-0432.CCR-06-3067
Palmela C, Velho S, Agostinho L, Branco F, Santos M, Santos MP, Oliveira MH, Strecht J, Maio R, Cravo M, Baracos VE (2017) Body composition as a prognostic factor of neoadjuvant chemotherapy toxicity and outcome in patients with locally advanced gastric cancer. J Gastric Cancer 17(1):74–87. https://doi.org/10.5230/jgc.2017.17.e8
Shachar SS, Deal AM, Weinberg M, Williams GR, Nyrop KA, Popuri K, Choi SK, Muss HB (2017) Body composition as a predictor of toxicity in patients receiving anthracycline and taxane-based chemotherapy for early-stage breast cancer. Clin Cancer Res 23(14):3537–3543. https://doi.org/10.1158/1078-0432.CCR-16-2266
Dolan RD, Almasaudi AS, Dieu LB, Horgan PG, McSorley ST, McMillan DC (2019) The relationship between computed tomography-derived body composition, systemic inflammatory response, and survival in patients undergoing surgery for colorectal cancer. J Cachexia Sarcopenia Muscle 10(1):111–122. https://doi.org/10.1002/jcsm.12357
Zhuang CL, Huang DD, Pang WY, Zhou CJ, Wang SL, Lou N, Ma LL, Yu Z, Shen X (2016) Sarcopenia is an independent predictor of severe postoperative complications and long-term survival after radical gastrectomy for gastric cancer: analysis from a large-scale cohort. Medicine 95(13):e3164. https://doi.org/10.1097/MD.0000000000003164
da Silva JR Jr, Wiegert EVM, Oliveira L, Calixto-Lima L (2019) Different methods for diagnosis of sarcopenia and its association with nutritional status and survival in patients with advanced cancer in palliative care. Nutrition 60:48–52. https://doi.org/10.1016/j.nut.2018.09.003
Mintziras I, Miligkos M, Wachter S, Manoharan J, Maurer E, Bartsch DK (2018) Sarcopenia and sarcopenic obesity are significantly associated with poorer overall survival in patients with pancreatic cancer: Systematic review and meta-analysis. Int J Surg 59:19–26. https://doi.org/10.1016/j.ijsu.2018.09.014
Fattouh M, Chang GY, Ow TJ, Shifteh K, Rosenblatt G, Patel VM, Smith RV, Prystowsky MB, Schlecht NF (2019) Association between pretreatment obesity, sarcopenia, and survival in patients with head and neck cancer. Head Neck 41(3):707–714. https://doi.org/10.1002/hed.25420
Faramarzi E, Mahdavi R, Mohammad-Zadeh M, Nasirimotlagh B (2013) Validation of nutritional risk index method against patient-generated subjective global assessment in screening malnutrition in colorectal cancer patients. Chin J Cancer Res 25(5):544–548. https://doi.org/10.3978/j.issn.1000-9604.2013.10.04
Geisler JP, Linnemeier GC, Thomas AJ, Manahan KJ (2007) Nutritional assessment using prealbumin as an objective criterion to determine whom should not undergo primary radical cytoreductive surgery for ovarian cancer. Gynecol Oncol 106(1):128–131. https://doi.org/10.1016/j.ygyno.2007.03.008
Bozzetti F, Cozzaglio L, Biganzoli E, Chiavenna G, De Cicco M, Donati D, Gilli G, Percolla S, Pironi L (2002) Quality of life and length of survival in advanced cancer patients on home parenteral nutrition. Clin Nutr 21(4):281–288
Bozzetti F, Arends J, Lundholm K, Micklewright A, Zurcher G, Muscaritoli M, Espen (2009) ESPEN guidelines on parenteral nutrition: non-surgical oncology. Clin Nutr 28(4):445–454. https://doi.org/10.1016/j.clnu.2009.04.011
WHO (2007) https://www.who.int/cancer/publications/cancer_control_palliative/en/
Culine S, Chambrier C, Tadmouri A, Senesse P, Seys P, Radji A, Rotarski M, Balian A, Dufour P (2014) Home parenteral nutrition improves quality of life and nutritional status in patients with cancer: a French observational multicentre study. Support Care Cancer 22(7):1867–1874. https://doi.org/10.1007/s00520-014-2164-9
Senesse P, Tadmouri A, Culine S, Dufour PR, Seys P, Radji A, Rotarski M, Balian A, Chambrier C (2015) A prospective observational study assessing home parenteral nutrition in patients with gastrointestinal cancer: benefits for quality of life. J Pain Symptom Manag 49(2):183–191 e182. https://doi.org/10.1016/j.jpainsymman.2014.05.016
Cotogni P, De Carli L, Passera R, Amerio ML, Agnello E, Fadda M, Ossola M, Monge T, De Francesco A, Bozzetti F (2017) Longitudinal study of quality of life in advanced cancer patients on home parenteral nutrition. Cancer Med 6(7):1799–1806. https://doi.org/10.1002/cam4.1111
Vashi PG, Dahlk S, Popiel B, Lammersfeld CA, Ireton-Jones C, Gupta D (2014) A longitudinal study investigating quality of life and nutritional outcomes in advanced cancer patients receiving home parenteral nutrition. BMC Cancer 14:593. https://doi.org/10.1186/1471-2407-14-593
Chermesh I, Mashiach T, Amit A, Haim N, Papier I, Efergan R, Lachter J, Eliakim R (2011) Home parenteral nutrition (HTPN) for incurable patients with cancer with gastrointestinal obstruction: do the benefits outweigh the risks? Med Oncol 28(1):83–88. https://doi.org/10.1007/s12032-010-9426-2
Santarpia L, Bozzetti F (2018) Acute impact of home parenteral nutrition in patients with late-stage cancer on family caregivers: preliminary data. Support Care Cancer 26(2):667–671. https://doi.org/10.1007/s00520-017-3884-4
Funding
This work was supported by grants from the Ministry of Science and Technology (MOST108-2321-B-037-001, MOST107-2321-B-037-003, MOST107-2314-B-037-116, MOST107-2314-B-037-022-MY2, and MOST107-2314-B-037-023-MY2) and the Ministry of Health and Welfare (MOHW107-TDU-B-212-123006, MOHW107-TDU-B-212-114026B, MOHW108-TDU-B-212-133006, and MOHW109-TDU-B-212-134026), which were funded by the health and welfare surcharge of tobacco products. Further funding was obtained from Kaohsiung Medical University Hospital (KMUH108-8R34, KMUH108-8R35, KMUH108-8M33, KMUH108-8M35, KMUH108-8M36, KMUHS10801, KMUHSA10804, KMUHS10807, and KMUH-DK109005~3), the Center for Cancer Research (KMU-TC108A04), and the Cohort Research Center Grant (KMU-TC108B07). In addition, this study was supported by the Grant of Taiwan Precision Medicine Initiative, Academia Sinica, Taiwan, ROC.
Author information
Authors and Affiliations
Contributions
C.J. Ma conducted the treatment, interpreted the final results, and drafted the manuscript. C.W. Huang and Y.S. Yeh collected data. H.L. Tsai, W.C. Su, and T.K. Chang participated in data analysis. L.C. Sun and Y.L. Shih assisted in treatment. F.J. Yu and D.C. Wu assisted in data interpretation. J.Y. Wang was responsible for study design and coordination. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interest.
Ethical approval
The protocol was approved by the local ethics committees (KMUHIRB-2013-02-71) and was performed in accordance with Declaration of Helsinki of 1975, as revised in 1996.
Consent to participate
Written informed consent was obtained from patients before any study activities were performed.
Consent for publish
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ma, CJ., Huang, CW., Yeh, YS. et al. Supplemental home parenteral nutrition improved nutrition status with comparable quality of life in malnourished unresectable/metastatic gastric cancer receiving salvage chemotherapy. Support Care Cancer 29, 1977–1988 (2021). https://doi.org/10.1007/s00520-020-05687-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-020-05687-4