Abstract
Purpose
The purposes of this study were to assess the levels of symptom distress, body image, and epidermal growth factor receptor inhibitors (EGFRI)-associated health-related quality of life (QoL); identify the factors related to EGFRI-associated health-related QoL; and examine the differences in EGFRI-associated health-related QoL by grade of skin toxicity in mCRC patients receiving target therapy.
Methods
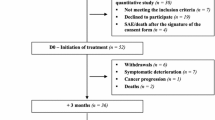
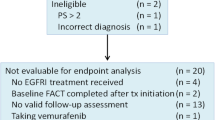
This cross-sectional study examined mCRC patients who received cetuximab-based target therapy from the oncology and CRC inpatient and outpatient departments of a medical center in northern Taiwan. Structured questionnaires were used to measure patients’ symptom distress, body image, and EGFRI-associated health-related QoL.
Results
Of the 111 mCRC patients studied, 79.2% reported acneiform eruption and 52.2% reported paronychia. The most common symptoms were dry skin and itching. Poor EGFRI-associated health-related QoL was associated with more symptom distress, more negative body image, a higher cumulative dose of target therapy, and being married; these factors explained 66.6% of the variance in EGFRI-associated health-related QoL.
Conclusion
Patient-specific skin care and emotional support are needed to relieve distressful dermatological symptoms and emotional distress during and post-treatment for mCRC.
Similar content being viewed by others
References
International agency for research on cancer (2019) Cancer fact sheets: colorectal cancer. Available from: https://gco.iarc.fr/today/fact-sheets-cancers? cancer=6&type=0&sex=0. Ministry of Health Welfare, Taiwan, ROC. 2017 statistics cause of death. Available from: http://www.doh.gov.tw. Accessed May 16, 2019
Taiwan cancer registry (2019) 2018 Annual Report. Available from: http://crs.cph.ntu.edu.tw/. Accessed May 16, 2019
Berretta M, Alessandrini L, De Divitiis C et al (2017) Serum and tissue markers in colorectal cancer: state of art. Crit Rev Oncol Hematol 111:103–116. https://doi.org/10.1016/j.critrevonc.2017.01.007
Stulhofer Buzina D, Martinac I, Ledic Drvar D et al (2016) Adverse reaction to cetuximab, an epidermal growth factor receptor inhibitor. Acta Dermatovenerol Croati 24:70–72
Busam KJ, Capodieci P, Motzer R, Kiehn T, Phelan D, Halpern AC (2001) Cutaneous side-effects in cancer patients treated with the antiepidermal growth factor receptor antibody C225. Br J Dermatol 144:1169–1176
D'Alessio A, Cecchini S, Di Mauro D et al (2016) Cutaneous toxicity from epidermal growth factor receptor inhibitors: would a subcutaneous desensitization be helpful? Tumori 102. https://doi.org/10.5301/tj.5000579
Folprecht G, Lutz MP, Schoffski P et al (2006) Cetuximab and irinotecan/5-fluorouracil/folinic acid is a safe combination for the first-line treatment of patients with epidermal growth factor receptor expressing metastatic colorectal carcinoma. Ann Oncol 17:450–456. https://doi.org/10.1093/annonc/mdj084
Giuliani J, Marzola M (2013) Skin rash during cetuximab treatment in advanced colorectal cancer: is age a clinical predictor? J Gastrointest Cancer 44:241–345. https://doi.org/10.1007/s12029-013-9485-7
Takada S, Sagawa T, Fujikawa K, Tahatsu K, Fukai Y, Hashishita H, Takahashi Y, Endo M (2018) Skin disorders and primary tumor location as prognostic factors in patients with metastatic colorectal cancer treated with cetuximab and chemotherapy. Asian Pac J Cancer Prev 19:2325–2330. https://doi.org/10.22034/APJCP.2018.19.8.2325
Peng Y, Li Q, Zhang J, Shen W, Zhang X, Sun C, Cui H (2019) Update review of skin adverse events during treatment of lung cancer and colorectal carcinoma with epidermal growth receptor factor inhibitors. Biosci Trends 12:537–552. https://doi.org/10.5582/bst.2018.01246
Charalambous A, Charalambous M (2016) “I lost my image, the image others know me by”: findings from a hermeneutic phenomenological study of patients living with treatment-induced cutaneous toxicities. Res Nurs Health 39:187–196. https://doi.org/10.1002/nur.21722
Charles C, Razavi D, Bungener C, Mateus C, Lanoy E, Verschoore M, Dauchy S, Robert C (2016) Impact of skin toxicities associated with targeted cancer therapies on body image: a prospective study. Clin Drug Investig 36:235–242. https://doi.org/10.1007/s40261-015-0373-8
De Tursi M, Zilli M, Carella C et al (2017) Skin toxicity evaluation in patients treated with cetuximab for metastatic colorectal cancer: a new tool for more accurate comprehension of quality of life impacts. OncoTargets Ther 16:3007–3015. https://doi.org/10.2147/OTT.S127795 eCollection 2017
Boone SL, Rademaker A, Liu D et al (2007) Impact and management of skin toxicity associated with anti-epidermal growth factor receptor therapy: survey results. Oncology 72:152–159
Karnofsky DA, Abelmann WH, Craver LF, Burchenal JH (1948) The use of the nitrogen mustards in the palliative treatment of carcinoma with particular reference to bronchogenic. Cancer 1:634–656
National comprehensive cancer network (2018) NCCN guidelines for patients: colon cancers 2018. Available From: http://www.nccn.org. Accessed May 16, 2019
McCorkle R, Young K (1978) Development of symptom distress scale. Cancer Nurs 1:373–378
Veale D, Eshkevari E, Kanakam N, Ellison N, Costa A, Werner T (2014) The appearance anxiety inventory: validation of a process measure in the treatment of body dysmorphic disorder. Behav Cogn Psychother 42:605–616. https://doi.org/10.1017/S1352465813000556
Wagner LI, Berg SR, Gandhi M, Hlubocky FJ, Webster K, Aneja M, Cella D, Lacouture ME (2013) The development of a functional assessment of cancer therapy (FACT) questionnaire to assess dermatologic symptoms associated with epidermal growth factor receptor inhibitors (FACT-EGFRI-18). Support Care Cancer 21:1033–1041. https://doi.org/10.1007/s00520-012-1623-4
National cancer institute (2010) Common terminology criteria for adverse events (CTCAE), Version 4.0. Available from: http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf. Accessed May 16, 2019
Bonomo P, Loi M, Desideri I et al (2017) Incidence of skin toxicity in squamous cell carcinoma of the head and neck treated with radiotherapy and cetuximab: a systematic review. Crit Rev Oncol Hematol 120:98–110. https://doi.org/10.1016/j.critrevonc.2017.10.011
Lacouture ME, Mitchell EP, Piperdi B, Pillai MV, Shearer H, Iannotti N, Xu F, Yassine M (2010) Skin toxicity evaluation protocol with panitumumab (STEPP), a phase II, open-label, randomized trial evaluating the impact of a pre-emptive skin treatment regimen on skin toxicities and quality of life in patients with metastatic colorectal cancer. J Clin Oncol 28:1351–1357. https://doi.org/10.1200/JCO.2008.21.7828
Lacouture ME, Maitland ML, Segaert S et al (2010) A proposed EGFR inhibitor dermatologic adverse event-specific grading scale from the MASCC skin toxicity study group. Support Care Cancer 8:509–522. https://doi.org/10.1007/s00520-009-0744-x
Fitzpatrick TB (1975) Sun and skin. Journal de Médecine Esthétique [in French] 2:33–34
Australian Radiation Protection and Nuclear Safety Agency (2007) Fitzpatrick skin phenotype. Available from: https://www.arpansa.gov.au/sites/default/files/legacy/pubs/RadiationProtection/FitzpatrickSkinType.pdf. Accessed May 16, 2019
Chan MWM, Shek SY, Yeung CK, Chan HH (2019) A prospective study in the treatment of lentigines in Asian skin using 532 nm picosecond Nd:YAG laser. Lasers Surg Med 51:767–773
Jo HC, Kim DY (2019) Correlation between light absorbance and skin color using fabricated skin phantoms with different colors. Lasers Med Sci 1–8. https://doi.org/10.1007/s10103-019-02888-0
Chang YL, Huang BS, Hung TM, Lin CY, Chen SC (2019) Factors influencing body image in posttreatment oral cavity cancer patients. Psychooncology 28:1127–1133. https://doi.org/10.1002/pon.5067
Chen SC, Huang BS, Hung TM et al (2019) Impact of a behavior change program and health education on social interactions in survivors of head and neck cancer: randomized controlled trial. Psychooncology 28:293–300. https://doi.org/10.1002/pon.4939
Thaler J, Karthaus M, Mineur L, Greil R, Letocha H, Hofheinz R, Fernebro E, Gamelin E, Baños A, Köhne CH (2012) Skin toxicity and quality of life in patients with metastatic cancer during first-line panitumumab plus FOLFIRI treatment in a single-arm phase II study. BMC Cancer 12:438. https://doi.org/10.1186/1471-2407-12-438
Dahlhoff M, Frances D, Kloepper JE, Paus R, Schäfer M, Niemann C, Schneider MR (2014) Overexpression of epigen during embryonic development induces reversible, epidermal growth factor receptor-dependent sebaceous gland hyperplasia. Mol Cell Biol 34:3086–3095. https://doi.org/10.1128/MCB.00302-14
Lacouture ME (2006) Mechanisms of cutaneous toxicities to EGFR inhibitors. Nat Rev Cancer 6:803–812
Galimont-Collen AF, Vos LE, Lavrijsen AP, Ouwerkerk J, Gelderblom H (2007) Classification and management of skin, hair, nail and mucosal side-effects of epidermal growth factor receptor (EGFR) inhibitors. Eur J Cancer 43:845–851
Esser P, Mehnert A, Johansen C, Hornemann B, Dietz A, Ernst J (2018) Body image mediates the effect of cancer-related stigmatization on depression: a new target for intervention. Psychooncology 27:193–198. https://doi.org/10.1002/pon.4494
Phelan SM, Griffin JM, Jackson GL, Zafar SY, Hellerstedt W, Stahre M, Nelson D, Zullig LL, Burgess DJ, van Ryn M (2013) Stigma, perceived blame, self-blame, and depressive symptoms in men with colorectal cancer. Psychooncology 22:65–73. https://doi.org/10.1002/pon.2048
Rosen AC, Case EC, Dusza SW, Balagula Y, Gordon J, West DP, Lacouture ME (2013) Impact of dermatologic adverse events on quality of life in 283 cancer patients: a questionnaire study in a dermatology referral clinic. Am J Clin Dermatol 14:327–333. https://doi.org/10.1007/s40257-013-0021-0
Joshi SS, Ortiz S, Witherspoon JN, Rademaker A, West DP, Anderson R, Rosenbaum SE, Lacouture ME (2010) Effects of epidermal growth factor receptor inhibitor-induced dermatologic toxicities on quality of life. Cancer 116:3916–3923. https://doi.org/10.1002/cncr.25090
Stanculeanu DL, Zob D, Toma OC, Georgescu B, Papagheorghe L, Mihaila RI (2017) Cutaneous toxicities of molecular targeted therapies. Maedica (Buchar) 12:48–54
Lee JJ, Kroshinsky D, Hoang MP (2017) Cutaneous reactions to targeted therapy. Am J Dermatopathol 39:67–82. https://doi.org/10.1097/DAD.0000000000000504
Davidson A, Williams J (2019) Factors affecting quality of life in patients experiencing facial disfigurement due to surgery for head and neck cancer. Br J Nurs 28:180–184. https://doi.org/10.12968/bjon.2019.28.3.180
Hirpara DH, Azin A, Mulcahy V et al (2019) The impact of surgical modality on self-reported body image, quality of life and survivorship after anterior resection for colorectal cancer–a mixed methods study. Can J Surg 62:1–8. https://doi.org/10.1503/cjs.014717
Clabbers JMK, Boers-Doets CB, Gelderblom H, Stijnen T, Lacouture ME, van der Hoeven K, Kaptein AA (2016) Xerosis and pruritus as major EGFRI-associated adverse events. Support Care Cancer 24:513–521. https://doi.org/10.1007/s00520-015-2781-y
Bensadoun RJ, Humbert P, Krutman J, Luger T, Triller R, Rougier A, Seite S, Dreno B (2013) Daily baseline skin care in the prevention, treatment, and supportive care of skin toxicity in oncology patients: recommendations from a multinational expert panel. Cancer Manag Res 5:401–408. https://doi.org/10.2147/CMAR.S52256 eCollection 2013
Acknowledgments
The authors gratefully acknowledge the patients who participated in the study. The authors also thank Convergence CT for the assistance of English editing.
Funding
Chang Gung Memorial Hospital, Grant/Award Number: CMRPG3G1591.
This study was supported by grant from Chang Gung Memorial Hospital (CMRPG3G1591) Research Program in Taiwan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study was approved by the Institutional Review Board of Chang Gung Medical Foundation Institutional Review Board in Taiwan, and a permission certificate was obtained (Number: 201701198B0).
Conflict of interest
The authors declare that they have no competing interests.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chiang, TY., Hsu, HC., Jane, SW. et al. EGFRI-associated health-related quality of life by severity of skin toxicity in metastatic colorectal cancer patients receiving epidermal growth factor receptor inhibitor target therapy. Support Care Cancer 28, 4771–4779 (2020). https://doi.org/10.1007/s00520-020-05321-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-020-05321-3