Abstract
Purpose
Tumor-related cancer pain often comprises mixed pain with both nociceptive and neuropathic components. Whether tumor-related cancer pain includes a neuropathic component impacts the therapeutic strategy. The aim of this cross-sectional study was to investigate the usefulness of two screening tools for neuropathic pain, painDETECT and Self-Report Leeds Assessment of Neuropathic Symptoms and Signs (S-LANSS), in identifying the neuropathic component of mixed pain among patients with tumor-related cancer pain.
Method
This cross-sectional study recruited consecutive inpatients and outpatients at a single site. The diagnostic accuracy of painDETECT and S-LANSS was evaluated using receiver operating characteristic curve analysis and classification probability.
Results
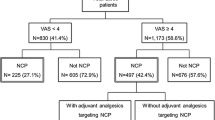
Of the study group, 106 patients had tumor-related cancer pain. Analyses of the nociceptive and mixed pain groups (n = 104) showed that neither painDETECT nor S-LANSS had satisfactory areas under the curve (AUCs) for identifying the neuropathic component of mixed pain (0.59 for painDETECT and 0.56 for S-LANSS). By pain intensity, the AUC for painDETECT was significantly higher in the mild pain group than in the moderate or severe pain group (0.77 vs. 0.43, P = 0.002). All parameters of classification probability for both tools were higher in the mild pain group than in the moderate or severe pain group.
Conclusions
painDETECT and S-LANSS could not identify the neuropathic component of mixed pain among patients with tumor-related cancer pain, especially when pain was moderate or severe. Contrarily, these screening tools might be useful for identifying the neuropathic component of mixed pain for mild pain.

Similar content being viewed by others
References
Bouhassira D, Luporsi E, Krakowski I (2017) Prevalence and incidence of chronic pain with or without neuropathic characteristics in patients with cancer. Pain 158:1118–1125
Garcia de Paredes ML, del Moral Gonzalez F, Martinez del Prado P, Marti Ciriquian JL, Enrech Frances S, Cobo Dols M, Esteban Gonzalez E, Ortega Granados AL, Majem Tarruella M, Cumplido Buron JD, Gasco Hernandez A, Lopez Miranda E, Ciria Santos JP, de Castro Carpeno FJ (2011) First evidence of oncologic neuropathic pain prevalence after screening 8615 cancer patients. Results of the On study. Ann Oncol 22:924–930
Bennett MI, Rayment C, Hjermstad M, Aass N, Caraceni A, Kaasa S (2012) Prevalence and aetiology of neuropathic pain in cancer patients: a systematic review. Pain 153:359–365
Mulvey MR, Boland EG, Bouhassira D, Freynhagen R, Hardy J, Hjermstad MJ, Mercadante S, Perez C, Bennett MI (2017) Neuropathic pain in cancer: systematic review, performance of screening tools and analysis of symptom profiles. Br J Anaesth 119:765–774
Kerba M, Wu JSY, Duan Q, Hagen NA, Bennett MI (2010) Neuropathic pain features in patients with bone metastases referred for palliative radiotherapy. J Clin Oncol 28:4892–4897
Pina P, Sabri E, Lawlor PG (2015) Characteristics and associations of pain intensity in patients referred to a specialist cancer pain clinic. Pain Res Manag 20:249–254
Reis-Pina P, Acharya A, Lawlor PG (2018) Cancer pain with a neuropathic component: a cross-sectional study of its clinical characteristics, associated psychological distress, treatments, and predictors at referral to a cancer pain clinic. J Pain Symptom Manag 55:297–306
Brunelli C, Bennett MI, Kaasa S, Fainsinger R, Sjogren P, Mercadante S, Lohre ET, Caraceni A (2014) Classification of neuropathic pain in cancer patients: a Delphi expert survey report and EAPC/IASP proposal of an algorithm for diagnostic criteria. Pain 155:2707–2713
Perez C, Sanchez-Martinez N, Ballesteros A, Blanco T, Collazo A, Gonzalez F, Villoria J (2015) Prevalence of pain and relative diagnostic performance of screening tools for neuropathic pain in cancer patients: a cross-sectional study. Eur J Pain 19:752–761
Mercadante S, Gebbia V, David F, Aielli F, Verna L, Casuccio A, Porzio G, Mangione S, Ferrera P (2009) Tools for identifying cancer pain of predominantly neuropathic origin and opioid responsiveness in cancer patients. J Pain 10:594–600
Potter J, Higginson IJ, Scadding JW, Quigley C (2003) Identifying neuropathic pain in patients with head and neck cancer: use of the Leeds Assessment of Neuropathic Symptoms and Signs Scale. J R Soc Med 96:379–383
Hardy J, Quinn S, Fazekas B, Agar M, Currow D (2013) Can the LANSS scale be used to classify pain in chronic cancer pain trials? Support Care Cancer 21:3387–3391
Tzamakou E, Petrou A, Tefa L, Siafaka V, Laou E, Tzimas P, Pentheroudakis G, Papadopoulos G (2018) Detection of neuropathic pain in end-stage cancer patients: diagnostic accuracy of two questionnaires. Pain Pract 18:768–776
Rayment C, Hjermstad MJ, Aass N, Kaasa S, Caraceni A, Strasser F, Heitzer E, Fainsinger R, Bennett MI (2013) Neuropathic cancer pain: prevalence, severity, analgesics and impact from the European Palliative Care Research Collaborative-Computerised Symptom Assessment study. Palliat Med 27:714–721
Swarm RA, Abernethy AP, Anghelescu DL, Benedetti C, Buga S, Cleeland C, Deleon-Casasola OA, Eilers JG, Ferrell B, Green M, Janjan NA, Kamdar MM, Levy MH, Lynch M, McDowell RM, Moryl N, Nesbit SA, Paice JA, Rabow MW, Syrjala KL, Urba SG, Weinstein SM, Dwyer M, Kumar R (2013) Adult cancer pain. J Natl Compr Cancer Netw 11:992–1022
Freynhagen R, Baron R, Gockel U, Tolle TR (2006) painDETECT: a new screening questionnaire to identify neuropathic components in patients with back pain. Curr Med Res Opin 22:1911–1920
Bennett MI, Smith BH, Torrance N, Potter J (2005) The S-LANSS score for identifying pain of predominantly neuropathic origin: validation for use in clinical and postal research. J Pain 6:149–158
Matsubayashi Y, Takeshita K, Sumitani M, Oshima Y, Tonosu J, Kato S, Ohya J, Oichi T, Okamoto N, Tanaka S (2013) Validity and reliability of the Japanese version of the painDETECT questionnaire: a multicenter observational study. PLoS One 8:e68013
Isomura T, Sumitani M, Matsudaira K, Kawaguchi M, Inoue R, Hozumi J, Tanaka T, Oshima H, Mori K, Taketomi S, Inui H, Tahara K, Yamagami R, Hayakawa K (2017) Development of the Japanese version of the Leeds Assessment of the Neuropathic Symptoms and Signs Pain Scale: diagnostic utility in a clinical setting. Pain Pract 17:800–807
Inoue S, Taguchi T, Yamashita T, Nakamura M, Ushida T (2017) The prevalence and impact of chronic neuropathic pain on daily and social life: a nationwide study in a Japanese population. Eur J Pain 21:727–737
Moreton BJ, Tew V, das Nair R, Wheeler M, Walsh DA, Lincoln NB (2015) Pain phenotype in patients with knee osteoarthritis: classification and measurement properties of painDETECT and self-report Leeds assessment of neuropathic symptoms and signs scale in a cross-sectional study. Arthritis Care Res 67:519–528
DeLong ER, DeLong DM, Clarke-Pearson DL (1988) Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 44:837–845
Boland EG, Mulvey MR, Bennett MI (2015) Classification of neuropathic pain in cancer patients. Curr Opin Support Palliat Care 9:112–115
Acknowledgments
We thank Ms. Masako Ikeda and Ms. Sachiko Nagatsuma at the Department of Palliative medicine, National Cancer Center Hospital East for their secretarial support.
Funding
This work was supported by a grant from Daiwa Securities Health Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. The study was approved by the National Cancer Center’s institutional review board. All participants provided written informed consent.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Higashibata, T., Tagami, K., Miura, T. et al. Usefulness of painDETECT and S-LANSS in identifying the neuropathic component of mixed pain among patients with tumor-related cancer pain. Support Care Cancer 28, 279–285 (2020). https://doi.org/10.1007/s00520-019-04819-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-019-04819-9