Abstract
Objective
Although clinical guidelines recommend administration of pegfilgrastim 1–4 days after a myelosuppressive chemotherapy cycle to decrease the incidence of febrile neutropenia (FN), some physicians administer pegfilgrastim on the same day as chemotherapy administration. A novel on-body injector (OBI) that automatically delivers pegfilgrastim the day after chemotherapy is also available. Our objective was to estimate patient and physician preferences among the pegfilgrastim administration options.
Methods
We conducted a cross-sectional survey of patients receiving pegfilgrastim and physicians prescribing pegfilgrastim. Respondents’ preferences for pegfilgrastim administration options were elicited using direct elicitation; the relative importance of features associated with the options was estimated in a point-allocation exercise. Physicians considered two hypothetical patient profiles when completing the exercises.
Results
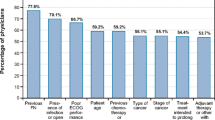
The samples included 200 patients and 200 physicians. Patients generally preferred the administration option with which they had experience. Among patients, 48.5% with previous in-clinic injections 24 hours after chemotherapy preferred this option; 56.8% with previous OBI administration preferred this option. For a clinically compromised patient, 37.5% of physicians preferred an in-clinic injection option; 49.5% preferred the OBI. For a less compromised patient, 55.5% preferred an in-clinic injection option; 28.0% preferred the OBI. Avoiding the need to return to the clinic was chosen most often as the most important treatment feature for patients and physicians.
Conclusions
Patients and physicians identified that returning clinic visits for pegfilgrastim administration may be burdensome. A potential solution to mitigate this burden is the OBI, which allows adherence to the labeled use of pegfilgrastim without return visits to the clinic.



Similar content being viewed by others
References
Crawford J, Dale DC, Kuderer NM et al (2008) Risk and timing of neutropenic events in adult cancer patients receiving chemotherapy: the results of a prospective nationwide study of oncology practice. J Natl Compr Cancer Netw 6:109–118
Vogel CL, Wojtukiewicz MZ, Carroll RR et al (2005) First and subsequent cycle use of pegfilgrastim prevents febrile neutropenia in patients with breast cancer: a multicenter, double-blind, placebo-controlled phase III study. J Clin Oncol 23:1178–1184
Neulasta® (pegfilgrastim) prescribing information (2016) Available from: http://pi.amgen.com/~/media/amgen/repositorysites/pi-amgen-com/neulasta/neulasta_pi_hcp_english.ashx. Accessed 9 December 2016
National Comprehensive Cancer Network (NCCN) (2015) Guidelines on myeloid growth factors, version 1. 2015. http://www.nccn.org/professionals/physician_gls/pdf/myeloid_growth.pdf. Accessed 1 September 2015
Smith TJ, Bohlke K, Lyman GH et al (2015) Recommendations for the use of WBC growth factors: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 33:3199–3212
Weycker D, Li X, Figueredo J et al (2016) Risk of chemotherapy-induced febrile neutropenia in cancer patients receiving pegfilgrastim prophylaxis: does timing of administration matter? Support Care Cancer 24:2309–2316
Marion S, Tzivelekis S, Darden C et al (2016) “same-day” administration of pegfilgrastim following myelosuppressive chemotherapy: clinical practice and provider rationale. Support Care Cancer 24:3889–3896
Neulasta Onpro healthcare provider instructions for use (2016) http://pi.amgen.com/united_states/neulasta/neulasta_ifu_hcp_pt_english.pdf. Accessed 10 November 2016
Berg MJ, Gross RA, Tomaszewski KJ et al (2008) Generic substitution in the treatment of epilepsy – case evidence of breakthrough seizures. Neurology 71:525–530
Devine S, Babrowicz J, Hahn R et al (2015) Intra-operative communication regarding neuromuscular blockade: a survey of anaesthesiologists and surgeons. J Anesth Clin Res 6:524–530
Hovinga CA, Asato MR, Manjunath R et al (2015) Association of non-adherence to antiepileptic drugs and seizures, quality of life, and productivity: survey of patients with epilepsy and physicians. Epilepsy Behav 13:316–322
Landy SH, Runken MC, Bell CF et al (2011) Assessing the impact of migraine onset on work productivity. J Occup Environ Med 53:74–81
Tomaszewski K, Deniz B, Tomanovich P et al (2012) Comparison of current US risk strategy to screen for hepatitis C virus with a hypothetical targeted birth cohort strategy. Am J Public Health 102:e101–e106
Joshi RS, Egbuna OI, Cairns AS et al (2016) Performance of the pegfilgrastim on-body injector as studied with placebo buffer in healthy volunteers. Curr Med Res Opin 5:1–21
Yang BB, Morrow PK, Wu X et al (2015) Comparison of pharmacokinetics and safety of pegfilgrastim administered by two delivery methods: on-body injector and manual injection with a prefilled syringe. Cancer Chemother Pharmacol 75:1199–1206
Acknowledgements
The authors gratefully acknowledge Jacob Garcia, MD, formerly of Amgen, Inc., for his clinical input on the study and the manuscript. Kate Lothman of RTI Health Solutions provided medical writing services, which were funded by Amgen, Inc.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
This work was supported by Amgen, Inc., Thousand Oaks, CA, United States. ABH, BM, MAP, DW, and JAK are employees of RTI Health Solutions, which received research funding from Amgen, Inc. MB and DC are employees and stockholders of Amgen, Inc.
Statement of human rights
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
ESM 1
(DOCX 42 kb)
Rights and permissions
About this article
Cite this article
Brett Hauber, A., Mange, B., Price, M.A. et al. Administration options for pegfilgrastim prophylaxis: patient and physician preferences from a cross-sectional survey. Support Care Cancer 26, 251–260 (2018). https://doi.org/10.1007/s00520-017-3841-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-017-3841-2