Abstract
Purpose
Chemotherapy/radiotherapy-induced nausea and vomiting (CINV/RINV) can affect half of oncology patients, significantly impacting daily life. Nausea without vomiting has only recently been thought of as a condition in its own right. As such, the incidence of nausea is often underestimated. This survey investigated the incidence and impact of CINV/RINV in patients compared with estimations of physicians/oncology nurses to determine if there is a perceptual gap between healthcare professionals and patients.
Methods
An online research survey of physicians, oncology nurses and patients was conducted across five European countries. Participants had to have experience prescribing/recommending or have received anti-emetic medication for CINV/RINV treatment. Questionnaires assessed the incidence and impact of CINV/RINV, anti-emetic usage and compliance, and attribute importance of anti-emetic medication.
Results
A total of 947 (375 physicians, 186 oncology nurses and 386 patients) participated in this survey. The incidence of nausea was greater than vomiting: 60 % of patients reported nausea alone, whereas 18 % reported vomiting. Physicians and oncology nurses overestimated the incidence of CINV/RINV but underestimated its impact on patients’ daily lives. Only 38 % of patients reported full compliance with physicians’/oncology nurses’ guidelines when self-administering anti-emetic medication. Leading factors for poor compliance included reluctance to add to a pill burden and fear that swallowing itself would induce nausea/vomiting.
Conclusions
There is a perceptual gap between healthcare professionals and patients in terms of the incidence and impact of CINV/RINV. This may lead to sub-optimal prescription of anti-emetics and therefore management of CINV/RINV. Minimising the pill burden and eliminating the requirement to swallow medication could improve poor patient compliance with anti-emetic regimens.
Similar content being viewed by others
Introduction
Nausea and vomiting induced by treatment are estimated, on any 1 day in routine practice, to affect 35–50 % of patients undergoing chemotherapy and/or radiotherapy [1]. One study found that this had a significant impact on quality of life in approximately 40 % of patients affected [2, 3]. Several studies have reported that the incidence of both nausea and vomiting is higher 2–5 days following chemotherapy/radiotherapy (the delayed phase) than it is during the first 24 h (the acute phase) [4, 5]. Although significant progress has been made in the prevention and control of chemotherapy/radiotherapy-induced nausea and vomiting (CINV/RINV) [6], a recent cross-sectional study in general hospitals revealed that the incidence of acute and delayed-phase vomiting was 12 and 23 % and nausea was 39 and 68 %, respectively [7].
In patients where the risk of nausea and vomiting (emesis) is regarded as high (highly emetogenic chemotherapy [HEC]) (>90 %) or moderate (moderately emetogenic chemotherapy [MEC]) (30–90 %), clinical research suggests that the optimal treatment to prevent CINV/RINV should associate a corticosteroid with a selective 5-HT3 receptor antagonist [8–12]. Dexamethasone is strongly recommended with a 5-HT3 antagonist (palonosetron or ondansetron) and an Nk1 receptor antagonist in high emetogenic chemotherapy regimens. Treatment should be administered at least 30 min prior to initiation of chemotherapy or radiotherapy and should continue throughout the delayed phase. However, many patients do not adhere to their regimen. Chan et al. [13] reported that 42 % of breast cancer patients are non-adherent to their post-chemotherapy anti-emetic treatment. Patients may also feel regular self-administration of anti-emetics unnecessary when not experiencing symptoms and may then find it difficult to gain control once symptoms present.
Nausea is reported more frequently by patients than emesis [5, 14]. However, nausea is a subjective experience and, unlike vomiting, difficult to define and quantify [15]. Nausea is often not regarded by patients and carers as a condition in itself but more frequently thought of as either a precursor or after-effect of vomiting [16]. It is now accepted that nausea is a distinct phenomenon, with its own mechanism of action, grades of severity and approach to treatment [16].
Several studies have highlighted how physicians and oncology nurses often underestimate the extent to which patients suffer from delayed CINV/RINV [5, 17–19]. These discrepancies can cause sub-optimal adherence by physicians to current CINV/RINV international guidelines when making prescribing decisions around the use of anti-emetics [20–23]. In addition, some patients can be reluctant to report symptoms of CINV/RINV, having the belief that these are part of the treatment process that they must endure. One study observed that approximately one third of patients expressed the desire ‘to be strong by not complaining’ and another that patients fear that complaints may lead to changes in their specific cancer therapy, impacting the chances of a cure [24, 25]. The differences observed in attitudes and experience of nausea and vomiting by physicians, nurses and patients suggest that there is a perceptual gap in the incidence and impact of CINV/RINV. Thus, it is likely that a significant proportion of patients suffer the consequences of CINV/RINV unnecessarily, impacting survival benefit and quality of life. This may be compounded by a lack of adherence of patients to their prescribed anti-emetic regimens, with two thirds of oncology patients keen to limit the amount of medication that they take [24].
We developed and utilised an observational, multinational, online survey in order to investigate the perceptions of CINV/RINV and understand what drives the use of anti-emetics. Oncology physicians, oncology nurses and patients in five European countries were surveyed to assess the impact of CINV/RINV on patients’ lives and gain an insight into their anti-emetic usage and satisfaction.
Methods
Participants
Inclusion criteria for physicians and nurses specialised in oncology were that they had been qualified for 3–35 years and were responsible for anti-emetic prescribing decisions/recommendations in cases of CINV/RINV (to a minimum of 20 and 10 patients per month for CINV prevention and CINV treatment, respectively). Physicians and nurses were verified as fully qualified and practising within their designated oncology role. Inclusion criteria for patients with cancer (irrespective of tumour type or disease stage) were that they were aged 18 years or over and had received chemotherapy and/or radiotherapy within the previous 24 months and had received anti-emetics for the treatment or prevention of CINV/RINV.
Participants were sourced from those already registered with patient and healthcare professionals’ survey panels and were invited to take part via email. Some physicians and oncology nurses were invited via telephone and patients via their doctor. Respondents were recruited in five European countries (France, Germany, Italy, Spain and the UK). Ethical and legal considerations of this survey are described in Online Resource 1.
Questionnaire design and outcomes
Initial wording for questions and multiple-answer options for this cross-sectional multinational survey were developed by the authors, including a representative of the study sponsor.
The questionnaire was piloted and adapted in the UK; five patients, two physicians and one oncology nurse completed the questionnaire and were subsequently interviewed for feedback, which was used to refine the wording of the questionnaire. Questionnaires were translated into the languages of the four other European countries for participants’ understanding. Copies of the final questionnaires are presented in Online Resources 2 and 3.
The questionnaire included consent and assessment of eligibility and was divided into eight sections with the following outcomes: eligibility, attitude and use of anti-emetic medications for CINV/RINV, incidence of CINV/RINV, impact of CINV/RINV on patient quality of life, compliance with anti-emetic regimens, patient assessment and communication, attribute importance, and demographics and classification. Respondents selected predefined answers, responded to open-ended questions or rated statements based on their opinions using a Likert scale [26]. Questionnaires were distributed 9 June–8 August 2014, inclusive.
Statistical analysis
Sample size was based on feasibility and was considered sufficient to demonstrate statistical significance at the aggregated country level by maintaining an error variance of below ±10 %. Descriptive summary statistics, including mean values and percentages, were used to summarise the data. Data from respondents in the different countries were pooled to analyse findings across the European population. Patient-physician and patient-oncology nurse discussions in the UK were compared with the rest of the European countries using 95 % confidence intervals. The Student’s t test was used to test for a perceptual gap between physicians, oncology nurses and patients.
Results
Survey population
The survey population comprised 947 respondents. A total of 386 patients were enrolled: 79 from Italy, 78 from the UK, 77 from France and 76 each from Germany and Spain. In total, 375 oncologists were enrolled, 75 from each European country surveyed, and 186 oncology nurses, 40 each from Germany, Italy and Spain, 35 from France and 31 from the UK.
Incidence and impact of nausea and vomiting
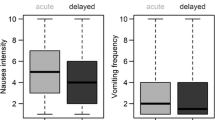
Of the total number of patients, 60 % (233/386) reported experiencing nausea only, 4 % (17/386) experienced vomiting only following their most recent cycle of cancer therapy and 14 % (55/386) of patients reported experiencing nausea in combination with vomiting. Therefore, nausea was reported by 74 % of patients and vomiting by a total of 18 %. The incidence of nausea and vomiting was higher in the acute phase than in the delayed phase: 55 and 15 % of patients reported acute-phase nausea or vomiting, respectively; 37 and 6 % reported delayed-phase nausea and vomiting, respectively (Table 1). Physicians and oncology nurses estimated the incidence of nausea and vomiting to be higher than patients recalled following their last round of chemotherapy and/or radiotherapy (Table 1). In addition, physicians and oncology nurses’ estimates of the incidence of nausea and vomiting in the acute phase were similar to their estimates of incidence in the delayed phase, despite patients recalling an improvement of symptoms in the delayed phase (Table 1).
Patients perceived the impact of nausea on their daily lives to be similar to the impact of vomiting. Mean impact scores (where 1 was minor impact and 10 was major impact) for chemotherapy-induced nausea and chemotherapy-induced vomiting were 4.6 and 5.3 in mild cases and 6.7 and 6.8 in moderate cases, respectively. Mean impact scores for radiotherapy-induced nausea and radiotherapy-induced vomiting were 5.2 and 5.7 for mild cases and 6.8 and 7.0 for moderate cases, respectively (Fig. 1). Physicians and oncology nurses’ perceptions of the impact of CINV/RINV on daily life were lower than reported by patients. This difference achieved statistical significance for mild and moderate-intensity CINV/RINV (p < 0.05), with the exception of physicians’ estimates of the impact of moderate chemotherapy-induced vomiting (p = 0.07). There was no statistical significance in the difference between physicians and oncology nurses’ perceptions and impact scores reported by patients for severe CINV/RINV (p > 0.05). Overall, 107/386 (28 %) patients felt that oncologists underestimated the impact of CINV/RINV. Most patients (260/386 [67 %]) responded ‘neither’ when asked if they felt that their doctor underestimated or overestimated the impact of nausea and vomiting on their daily lives. Few patients felt that oncologists overestimated the impact of CINV/RINV (19/386 [5 %]).
Mean rating of the impact that nausea/vomiting has on patients’ daily lives. Data presented are mean ratings on a scale of 1 to 10, where 1 is minor impact and 10 is major impact (physicians, n = 375; oncology nurses, n = 186; patients, n = 386). In mild and moderate settings, the mean score differences between physicians and oncology nurses’ and patients’ perceptions are statistically significant, with the exception of physicians’ estimates of the impact of moderate-chemotherapy-induced vomiting. There was no statistically significant difference between physicians and oncology nurses’ estimates of the impact of severe chemotherapy/radiotherapy-induced nausea and vomiting compared with patients’ perceptions. p values were calculated using Student’s t test
Control of nausea and vomiting
Approximately three quarters of physicians (285/375 [76 %]) prescribed guideline-suggested prophylaxis for HEC where the emetogenic potential of chemotherapy was high. This decreased to 56/375 (15 %) when emetogenic potential was moderate (MEC). Most physicians (322/375 [86 %]) prescribed no or minimal anti-emetic medication for low emetogenic chemotherapy (LEC) treatment. One quarter (94/375 [25 %]) of physicians reported poor side effects/tolerability and 66/375 (18 %) reported cost as being key factors making them reluctant to prescribe prophylactic anti-emetic medication where therapy has significant emetogenic potential.
Physicians, oncology nurses and patients were asked to rank in order the importance of seven treatment goals when deciding upon anti-emetic medication to reduce CINV/RINV using a Likert scale where 1 was most desirable and 7 the least (Table 2). Physicians and oncology nurses both ranked reducing episodes of vomiting as more important than reducing episodes of nausea (mean values, physicians 2.4 versus 2.9; oncology nurses 2.6 versus 3.0, respectively). Patients’ prioritisation was similar for reducing episodes of vomiting and reducing episodes of nausea (mean values, 3.4 and 3.3, respectively).
Half the patients surveyed (197/386 [51 %]) were given anti-emetic medication on the day of treatment at the hospital for the treatment of acute CINV/RINV; 58/197 (29 %) of these received treatment less than 30 min prior to chemotherapy/radiotherapy. Of the total number of patients, 329/386 (85 %) were given anti-emetic medication for treatment of delayed CINV/RINV at home. The most common form of administration was oral: a pill/tablet (182/329 [55 %]), an orodispersable film (73/329 [22 %]) or syrup (44/329 [13 %]). Of the 45/386 (12 %) patients that were not given medication at home, 13/45 (29 %) were told that it was because they were unlikely to experience CINV/RINV and 14/45 (31 %) did not know why.
Visual inspection of the data showed that in the UK, oncology nurses had a greater role in discussing CINV/RINV with patients than in all other European countries surveyed and that more patients discussed this with their oncology nurse: 22/78 (28 %), UK, compared with 19/76 (25 %), Spain, 16/77 (21 %), France, 15/76 (20 %), Germany, and 14/79 (18 %), Italy. Forty-six of 78 (59 %) patients in the UK reported discussing CINV/RINV with their physician whereas patient–physician discussions were more frequent than in other European countries: 55/79 (70 %), Italy, 54/77 (70 %), France, 50/76 (66 %), Germany, and 50/76 (66 %), Spain. However, analysis of 95 % confidence intervals demonstrated that there was no statistically significant difference in patient–physician and patient–oncology nurse discussions in the UK compared with the other European countries tested.
Similar proportions of all physicians, oncology nurses and patients surveyed (275/375 [73 %], 124/186 [67 %] and 263/386 [68 %], respectively) rated the interaction between patient and care team as highly or moderately structured. The remaining respondents felt that it was either loosely structured or non-existent (Fig. 2). Furthermore, 100/375 (27 %) physicians and 61/186 (33 %) oncology nurses stated that there was no patient assessment during the 5-day period following chemotherapy/radiotherapy, which was confirmed by half of the patients (Fig. 3). The main reason for not reporting CINV/RINV was assigned to acceptance and belief that it had to be tolerated; this option was selected by 196/386 (51 %) patients. This was estimated by 224/375 (60 %) physicians and 139/186 (75 %) nurses to be the main reason for not reporting CINV/RINV.
Type of interaction between patients and care team during the 5-day period post-administration of chemotherapy/radiotherapy. Data are presented as the percentage of physicians (n = 375), oncology nurses (n = 186) and patients (n = 386) who rated the interaction between the patient and care team as highly structured, moderately structured, loosely structured or unstructured
Assessment of patients during the 5-day period post-administration of chemotherapy/radiotherapy. Data are presented as the percentage of physicians (n = 375) and oncology nurses (n = 186) who selected predefined answers that best described the assessment of their patients. Patient data (n = 386) are presented as the percentage of patients contacted by their care team in the 5 days immediately following administration of chemotherapy/radiotherapy
Investigations into satisfaction with anti-emetic regimens revealed that 80/386 (21 %) patients considered the anti-emetics prescribed to them on their first cycle of chemotherapy/radiotherapy to be insufficient; a mean of 2.4 changes to medication was necessary to control nausea and vomiting. Physicians estimated that the most frequently administered anti-emetics were dexamethasone (mean estimate of 59 % of patients), ondansetron (47 %), metoclopramide (35 %) and aprepitant (25 %). Nine per cent (36/386) of patients felt their current anti-emetic medication to be insufficient and described themselves as dissatisfied.
Patient adherence with anti-emetic regimen
Physicians and oncology nurses estimated that approximately two thirds of patients (61 and 66 %, respectively) adhered fully to their prescribed anti-emetic regimen at home. However, only 145/386 (38 %) patients recalled always taking their anti-emetic medication according to their physicians’ or oncology nurses’ instructions (frequency/timing of self-administration). The reason selected by most physicians (155/375 [41 %]), oncology nurses (105/186 [56 %]) and patients (67/241 [28 %]) for poor adherence was ‘not accepting the need to take medication until actually feeling sick’. In addition, physicians, oncology nurses and patients rated the reluctance to add to an overall pill burden and a fear that the action of swallowing itself would induce nausea/vomiting as discouraging factors when taking anti-emetic medication (Table 3).
Discussion
This survey investigated the incidence and impact of CINV/RINV and explored differences in opinions and management between physicians’, oncology nurses’ and patients’ experience, through an online questionnaire. Key findings included that patients reported the incidence of nausea as being greater than vomiting and that the impact that nausea has on patients’ daily lives is as severe as vomiting. This survey also demonstrated that there is a perceptual gap between physicians and oncology nurses and patients: physicians and nurses overestimated the incidence of CINV/RINV but underestimated the impact that this had on patients’ daily lives.
Previous studies showed that the overall incidence of chemotherapy/radiotherapy-induced nausea is more frequent than vomiting, as demonstrated in the current study [5, 14, 27]. Unlike previous data [4, 5], data generated in this study indicated that the incidence of acute CINV/RINV was greater than delayed CINV/RINV. This could be attributed to the fact that these previous studies refer specifically to patients’ first cycle of chemotherapy, whereas this study refers to patients’ most recent cycle of treatment. As such, an element of anticipatory emesis, which is a learned, psychological response that develops following exposure to repeated chemotherapy cycles, may have been incorporated [28]. Patients in this survey recalled that nausea and vomiting both improved during the delayed phase; however, physicians and oncology nurses overestimated the incidence, suggesting that they might have had preconceptions that the severity of acute CINV/RINV was sustained for the duration of the delayed phase. Patients whose CINV/RINV worsened in the delayed phase have reported that physicians did not titrate their medication to accommodate this, indicative of the difficulty of physicians to distinguish between the acute and delayed phases [7].
The patient experience can be exacerbated because of the negative impact of CINV/RINV on daily life and its effects on performing everyday tasks such as eating, sleeping and socialising [29]. Patients from this survey as well as from a previous study ranked the impact of nausea as being equally as severe as vomiting [30], which may partly be explained by patients often having difficulty describing nausea as it is a subjective symptom and not a quantifiable symptom like vomiting [29]. In addition, vomiting subsides once the patient has been sick, whereas there is little that can be done to ease nausea other than avoid its triggers [15]. This can be problematic as triggers include food and aromas, which are difficult to avoid [29].
When emetogenic potential of therapy was scored as high, the majority of physicians prescribed guideline-suggested prophylaxis for HEC. This decreased with emetogenic potential, and most physicians prescribed either minimum or no anti-emetic medication for LEC. This is likely to be compounded by the fact that a quarter of physicians were reluctant to prescribe anti-emetic medication because of their side effects. Thus, there is the potential for anti-emetic medications to be under-prescribed to patients whose cancer treatment is regarded as LEC or MEC.
Current guidelines state that antiemetic prevention should consist of a 5-HT3 receptor antagonist (such as palonosetron or ondansetron) in combination with dexamethasone for the treatment of acute CINV/RINV, with dexamethasone administration sustained for the duration of the delayed phase for MEC treatment. Combination should be supplemented with aprepitant throughout both phases for HEC treatment [10–12]. Dexamethasone is recommended for the treatment of acute CINV/RINV, but no medication is required for delayed CINV/RINV, following LEC therapy [11]. Nearly one third of patients receiving anti-emetic medication on the day of their treatment received it less than the 30 min prior to starting chemotherapy/radiotherapy contrary to recommendation in treatment guidelines [10–12]. Guideline-consistent CINV prophylaxis significantly improves CINV symptoms compared with guideline-inconsistent CINV prophylaxis [20, 23] indicating that anti-emetic treatments should be administered prophylactically to inhibit the initiation of nausea and vomiting. However, as data generated by this survey applied specifically to the patients’ most recent cycle of treatment, it may not necessarily have reflected management throughout multiple treatment cycles.
Physicians, oncology nurses and patients rated the structure of the interaction between the patient and the care team similarly following chemotherapy/radiotherapy. However, there appeared to be inter-country variation in the responsibility for discussing CINV/RINV with patients; in the UK, more patients discussed CINV/RINV with their oncology nurse, whereas in the other European countries surveyed, they did more so with their physicians, although this trend was not statistically significant. This may reflect the different roles of oncology nurses in the UK compared with other European countries; in the UK, nurses are often responsible for providing treatment and taking patient calls when they become unwell. In the rest of Europe, however, many patients are managed primarily by their physician.
Nearly two thirds of patients recalled not fully adhering to their anti-emetic medication. As results in this study were based on patient recall, actual adherence levels may be lower due to incorrect self-administration [31]. The main reasons selected for non-adherence were refusing to take medication until actually feeling sick and the perceived inevitability of CINV/RINV, suggesting that the majority of patients were unaware of the relationship between anti-emetic adherence and reduced incidence of CINV [23]. This highlights the importance of reassuring patients that they should take their anti-emetic treatment prophylactically rather than after the emergence of symptoms. Anti-emetic medication in the form of a pill/tablet was the most frequent formulation given to patients for the treatment of delayed CINV/RINV, but the reluctance to add to a pill burden and a fear that swallowing a pill/tablet will itself induce nausea and/or vomiting also contributed to poor adherence. Consequently, eliminating the requirement for swallowing a pill/tablet would be a desirable characteristic of anti-emetic medication and could aid poor patient adherence with anti-emetic regimens.
Several potential sources of bias were identified in the study design, which was enhanced to attenuate these. Selection bias was likely to impact our data: This was an online survey, and so, physicians, oncology nurses and, more notably, patients who have access to the Internet may not be fully representative of their entire demographic groups, especially elderly patients. To mitigate this, respondents were not subjected to an age limit, and patients were eligible regardless of their tumour type and disease stage in an attempt to target as wide a range of respondents as possible. Voluntary response bias may also have been a factor: Recipients were self-selected volunteers, and so, this bias may have led to over-representation of individuals with particularly strong opinions. We considered the whole patient population without stratification according to class of emetic potential regimen (high, moderate or low). Stratification would have led to smaller numbers of patients, making analyses less robust. Patients’ intention to please may have influenced our results, but this risk was minimised by respondents remaining anonymous so as not to damage the physician/patient relationship. The desire to be ‘strong by not complaining’ has been reported by patients in regard to CINV/RINV [24], and this should be considered when assessing the patient experience.
In conclusion, this study showed that there was a perceptual gap between the healthcare professionals (physicians and oncology nurses) who make anti-emetic prescribing decisions/recommendations and the patients who receive anti-emetic treatment in terms of CINV/RINV impact on their daily life. The severity and impact of nausea (without vomiting) on patients were greater than physicians and oncology nurses estimated. Physicians and nurses failed to distinguish between acute and delayed CINV/RINV, which could lead to poor management of delayed CINV/RINV. Physicians’ adherence to clinical guidelines when prescribing anti-emetic medication and targeting CINV/RINV prophylactically rather than retrospectively will improve CINV/RINV. Finally, the formulation of anti-emetic medication is important; minimising the pill burden and eliminating the requirement to swallow medication will improve patient adherence during treatment for CINV/RINV.
References
Grunberg S, Clark-Snow RA, Koeller J (2010) Chemotherapy-induced nausea and vomiting: contemporary approaches to optimal management. Proceedings from a symposium at the 2008 Multinational Association of Supportive Care in Cancer (MASCC) Annual Meeting. Support Care Cancer 18:S1–S10
Lindley CM, Hirsch JD, O’Neill CV, Transau MC, Gilbert CS, Osterhaus JT (1992) Quality of life consequences of chemotherapy-induced emesis. Qual Life Res 1:331–340
Fernández-Ortega P, Caloto MT, Chirveches E, Marquilles R, Francisco JS, Quesada A, Suárez C, Zorrilla I, Gómez J, Zabaleta P, Nocea G, Llombart-Cussac A (2012) Chemotherapy-induced nausea and vomiting in clinical practice: impact on patients’ quality of life. Support Care Cancer 20:3141–3148
Glaus A, Knipping C, Morant R, Böhme C, Lebert B, Beldermann F, Glawogger B, Fernandez-Ortega P, Hüsler A, Deuson R (2004) Chemotherapy-induced nausea and vomiting in routine practice: a European perspective. Support Care Cancer 12:708–715
Grunberg SM, Deuson RR, Mavros P, Geling O, Hansen M, Cruciani G, Daniele B, De Pouvourville G, Rubenstein EB, Daugaard G (2004) Incidence of chemotherapy-induced nausea and emesis after modern antiemetics. Cancer 100:2261–2268
Bhandari PR (2012) Recent advances in pharmacotherapy of chemotherapy-induced nausea and vomiting. J Adv Pharm Technol Res 3:202–209
Hilarius DL, Kloeg PH, van der Wall E, van den Heuvel JJ, Gundy CM, Aaronson NK (2012) Chemotherapy-induced nausea and vomiting in daily clinical practice: a community hospital-based study. Support Care Cancer 20:107–117
Jordan K, Sippel C, Schmoll HJ (2007) Guidelines for antiemetic treatment of chemotherapy-induced nausea and vomiting: past, present, and future recommendations. Oncologist 12:1143–1150
Roila F, Herrstedt J, Aapro M, Gralla RJ, Einhorn LH, Ballatori E, Bria E, Clark-Snow RA, Espersen BT, Feyer P, Grunberg SM, Hesketh PJ, Jordan K, Kris MG, Maranzano E, Molassiotis A, Morrow G, Olver I, Rapoport BL, Rittenberg C, Saito M, Tonato M, Warr D, ESMO/MASCC Guidelines Working Group (2010) Guideline update for MASCC and ESMO in the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting: results of the Perugia consensus conference. Ann Oncol 21(Suppl 5):v232–v243
Basch E, Hesketh PJ, Kris MG, Prestrud AA, Temin S, Lyman GH (2011) Antiemetics: American Society of Clinical Oncology clinical practice guideline update. J Oncol Pract 7:395–398
Jordan K, Gralla R, Jahn F, Molassiotis A (2014) International antiemetic guidelines on chemotherapy induced nausea and vomiting (CINV): content and implementation in daily routine practice. Eur J Pharmacol 722:197–202
MASCC (2013) MASCC/ESMO antiemetic guidelines. http://www.mascc.org/assets/Guidelines-Tools/mascc_guidlines_english_2014.pdf. Accessed 19 December 2014
Chan A, Low XH, Yap KY (2012) Assessment of the relationship between adherence with antiemetic drug therapy and control of nausea and vomiting in breast cancer patients receiving anthracycline-based chemotherapy. J Manag Care Pharm 18:385–294
Molassiotis A, Saunders MP, Valle J, Wilson G, Lorigan P, Wardley A, Levine E, Cowan R, Loncaster J, Rittenberg C (2008) A prospective observational study of chemotherapy-related nausea and vomiting in routine practice in a UK cancer centre. Support Care Cancer 16:201–208
Glare P, Miller J, Nikolova T, Tickoo R (2011) Treating nausea and vomiting in palliative care: a review. Clin Interv Aging 6:243–259
Grunberg S (2012) Patient-centered management of chemotherapy-induced nausea and vomiting. Cancer Control 19:10–15
Liau CT, Chu NM, Liu HE, Deuson R, Lien J, Chen JS (2005) Incidence of chemotherapy-induced nausea and vomiting in Taiwan: physicians’ and nurses’ estimation vs. patients’ reported outcomes. Support Care Cancer 13:277–286
Erazo Valle A, Wisniewski T, Figueroa Vadillo JI, Burke TA, Martinez Corona R (2006) Incidence of chemotherapy-induced nausea and vomiting in Mexico: healthcare provider predictions versus observed. Curr Med Res Opin 22:2403–2410
Bash E (2010) The missing voice of patients in drug-safety reporting. N Engl J Med 362:865–869
Aapro M, Molassiotis A, Dicato M, Peláez I, Rodríguez-Lescure Á, Pastorelli D, Ma L, Burke T, Gu A, Gascon P, Roila F, PEER investigators (2012) The effect of guideline-consistent antiemetic therapy on chemotherapy-induced nausea and vomiting (CINV): the Pan European Emesis Registry (PEER). Ann Oncol 23:1986–1992
Burmeister H, Aebi S, Studer C, Fey MF, Gautschi O (2012) Adherence to ESMO clinical recommendations for prophylaxis of chemotherapy-induced nausea and vomiting. Support Care Cancer 20:141–147
Gomez DR, Liao KP, Giordano S, Nguyen H, Smith BD, Elting LS (2013) Adherence to national guidelines for antiemesis prophylaxis in patients undergoing chemotherapy for lung cancer: a population-based study. Cancer 119:1428–1436
Gilmore JW, Peacock NW, Gu A, Szabo S, Rammage M, Sharpe J, Haislip ST, Perry T, Boozan TL, Meador K, Cao X, Burke TA (2014) Antiemetic guideline consistency and incidence of chemotherapy-induced nausea and vomiting in US community oncology practice: INSPIRE Study. J Oncol Pract 10:68–74
Salsman JM, Grunberg SM, Beaumont JL, Rogers M, Paul D, Clayman ML, Cella D (2012) Communicating about chemotherapy-induced nausea and vomiting: a comparison of patient and provider perspectives. J Natl Compr Canc Netw 10:149–157
Janelsins MC, Tejani MA, Kamen C, Peoples AR, Mustian KM, Morrow GR (2013) Current pharmacotherapy for chemotherapy-induced nausea and vomiting in cancer patients. Expert Opin Pharmacother 14:757–766
Likert R (1932) A technique for the measurement of attitudes. Arch Psychol 22:5–55
Roscoe JA, Morrow GR, Hickok JT, Stern RM (2000) Nausea and vomiting remain a significant clinical problem: trends over time in controlling chemotherapy-induced nausea and vomiting in 1413 patients treated in community clinical practices. J Pain Symptom Manage 20:113–121
Kamen C, Tejani MA, Chandwani K, Janelsins M, Peoples AR, Roscoe JA, Morrow GR (2014) Anticipatory nausea and vomiting due to chemotherapy. Eur J Pharmacol 722:172–179
Molassiotis A, Stricker CT, Eaby B, Velders L, Coventry PA (2008) Understanding the concept of chemotherapy-related nausea: the patient experience. Eur J Cancer Care (Engl) 17:444–453
Griffin AM, Butow PN, Coates AS, Childs AM, Ellis PM, Dunn SM, Tattersall MH (1996) On the receiving end. V: patient perceptions of the side effects of cancer chemotherapy in 1993. Ann Oncol 7:189–195
Jimmy B, Jose J (2011) Patient medication adherence: measures in daily practice. Oman Med J 26:155–159
Acknowledgments
This questionnaire was administered by the Healthcare Division of GfK NOP Limited and complied with Market, Opinion and Social Research Standards and Quality Assurance Standards. Derek Holley of GfK NOP Limited provided input into the design of the questionnaire. This study was sponsored by Norgine Ltd and opinions of oncology experts were sought when constructing questions and analysing data. Dr Ellen Robertshaw and Dr Justin Cook of Niche Science and Technology Ltd provided medical writing support, which was paid for by Norgine Ltd.
Conflict of interest
This study was funded by Norgine Ltd. Bharat Amlani is employed by Norgine Ltd. Cheryl Vidall is a consultant for Norgine Ltd. Florian Scotté has received honoraria from Norgine, has been a consultant/advisor for Merck and Vifor and has been on an advisory board for Prostrakan. Patrick Jahn has received renumeration from Helsinn, Norgine and Clinigen and been a consultant/advisor for Clinigen. Paz Fernández-Ortega and Diego Cortinovis have no disclosures to declare. Bharat Amlani has full control of all primary data and agrees to allow the journal to review the data if requested.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Online Resource 1
Summary of the ethical and legal considerations of the study (DOCX 20 kb)
Online Resource 2
Questionnaire distributed to physicians and oncology nurses (DOCX 71 kb)
Online Resource 3
Questionnaire distributed to patients (DOCX 74 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Vidall, C., Fernández-Ortega, P., Cortinovis, D. et al. Impact and management of chemotherapy/radiotherapy-induced nausea and vomiting and the perceptual gap between oncologists/oncology nurses and patients: a cross-sectional multinational survey. Support Care Cancer 23, 3297–3305 (2015). https://doi.org/10.1007/s00520-015-2750-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-015-2750-5