Abstract
Purpose
Oral mucositis (OM) is a side effect of intensive chemotherapy and radiation and has been reported to affect 75–100 % of hematopoietic stem cell transplantation (HSCT) recipients. The purpose of this study was to compare the incidence of OM in patients conditioned with myeloablative conditioning (MAC) to reduced-intensity conditioning (RIC) and to determine the effect of a new oral care protocol.
Methods
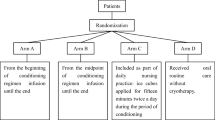
The study involved 171 HSCT recipients, with hematological malignancies transplanted between 2007 and 2011. Median age of the patients was 50 years (range 12–71). Ninety-nine (58 %) received RIC and 72 received MAC. Clinical features of OM were recorded from day −3 before to day +25 after HSCT using the World Health Organization (WHO) scoring system and the oral mucositis assessment score (OMAS).
Results
Overall, 87 % of the patients developed OM of any severity, which peaked on days 10–11. The mean WHO score was 1.7. In multivariate analysis, the severity of OM was associated with MAC (relative hazard (RH) 1.57, 95 % confidence interval (CI) 1.37–1.80, p < 0.001), all donor-recipient gender combinations except female-to-male (RH = 1.26, 95 % CI 1.10–1.4, p = 0.001), and early year of HSCT (RH = 0.84, 95%CI 0.7–0.96, p = 0.013). There was a correlation between long hospitalization and OM (day 15, r = 0.31, p < 0.001). There was a good correlation between the WHO and OMAS scoring systems for OM (r = 0.74, p < 0.001).
Conclusions
Oral mucositis was reduced in patients treated with RIC and in patients treated during recent years, when oral care was intensified. Increased scores of OM prolonged hospitalization.




Similar content being viewed by others
References
Heimdahl A, Johnson G, Danielsson K et al (1985) Oral condition of patients with leukemia and severe aplastic anemia. Oral Surg Oral Med Oral Pathol 60:498–504
Barett AP (1986) Oral complications in bone marrow transplantation. Aust N Z J Med 16:239–40
Blijlevens NMA, Donelly JP, De Pauw BE (2000) Mucosal barrier injury: biology, pathology, clinical counterparts and consequences of intensive treatment for heamatological malignancy: an overview. Bone Marrow Transplant 25:1269–1278
Schubert MM, Williams BE, Lloid ME, Donaldson G, Chapko MK (1992) Clinical assessment scale for the rating of oral mucosal changes associated with bone marrow transplantation. Development of an oral mucositis index. Cancer 69:2469–2477
Woo SB, Sonis ST, Monopoli MM, Sonis AL (1993) A longitudinal study of oral ulcerative mucositis in bone marrow transplant recipients. Cancer 72:1612–1617
Vagliano L, Feraut C, Gobetto G et al (2011) Incidence and severity of oral mucositis in patients undergoing haematopoietic SCT—results of a multicenter study. Bone Marrow Transplant 46:727–732
Mattsson T, Heimdahl A, Dahllöf G, Lönnqvist B, Nilsson B, Ringdén O (1991) Variables predicting oral mucosal lesions in allogeneic bone marrow recipients. Head Neck 13:224–229
Dahllöf G, Heimdahl A, Bolme P, Lönnqvist B, Ringdén O (1988) Oral condition in children treated with bone marrow transplantation. Bone Marrow Transplant 3:43–51
Sonis ST (2009) Mucositis: the impact, biology and therapeutic opportunities of oral mucositis. Oral Oncol 45:1015–1020
Donelly JP, Muus P, Schattenberg A, De Witte T, Horrevorts A, DePauw BE (1992) A scheme for daily monitoring of oral mucositis in allogenic BMT recipients. Bone Marrow Transplant 9:409–413
Tabbara I, Zimmerman K, Morgan C, Naleh Z (2002) Allogenic hematopoietic stem cell transplantation. Complications and results. Arch Intern Med 162:1558–1566
Vera-Llonch M, Oster G, Ford CM, Lu J, Sonis S (2007) Oral mucositis and outcomes of allogeneic hematopoietic stem cell transplantation in patients with hematologic malignancies. Support Care Cancer 15:491–496
Ringdén O, Le Blanc K (2005) Allogeneic hematopoietic stem cell transplantation: state of the art and new perspectives. APMIS 113:813–30
Ringdén O, Labopin M, Ehninger G et al (2009) Reduced intensity conditioning compared with myeloablative conditioning using unrelated donor transplant in patients with acute myeloid leukemia. J Clin Oncol 27:4570–4577
Giralt S, Estey E, Albitar M et al (1997) Engraftment of allogeneic hematopoietic progenitor cells with purine analogue-containing chemotherapy: harnessing graft-versus-leukemia without myeloablative therapy. Blood 89:4531–4536
McSweeney PA, Niederwieser D, Shizuru JA et al (2001) Hematopoietic cell transplantation in older patients with hematologic malignancies: replacing high-dose cytotoxic therapy with graft-versus-tumor effects. Blood 97:3390–3400
World Health Organization (1979) WHO handbook for reporting results for cancer treatment, vol 48. World Health Organization, Geneva, p 1
Sonis ST, Oster G, Fuchs H et al (2001) Oral mucositis and the clinical and economic outcomes of hematopoietic stem cell transplantation. J Clin Oncol 19:2201–2205
Svahn BM, Remberger M, Myrbäck KE et al (2002) Home care during the pancytopenic phase after allogeneic hematopoietic stem cell transplantation is advantageous compared with hospital care. Blood 100:4317–4324
Schaffer M, Aldener-Cannavá A, Remberger M, Ringdén O, Olerup O (2003) Roles of HLA-B, HLA-C and HLA-DPA1 incompatibilities in the outcome of unrelated stem-cell transplantation. Tissue Antigens 62:243–250
Storb R, Deeg HJ, Fisher L et al (1988) Cyclosporine vs methotrexate for graft-v-host disease prevention in patients given marrow grafts for leukemia: long-term follow-up of three controlled trials. Blood 71:293–298
Ringdén O, Remberger M, Persson U et al (1995) Similar incidence of graft-versus-host disease using HLA-A, -B and -DR identical unrelated bone marrow donors as with HLA-identical siblings. Bone Marrow Transplant 15:619–1625
Ringdén O, Uzunel M, Rasmusson I et al (2006) Mesenchymal stem cells for treatment of therapy-resistant graft-versus-host disease. Transplantation 81:1390–1397
Lundgren G, Wilczek H, Lönnqvist B, Lindholm A, Wahren B, Ringdén O (1985) Acyclovir prophylaxis in bone marrow transplant recipients. Scand J Infect Dis 47:137–144
National Cancer Institute (2003) Common terminology criteria for adverse events (CTCAE). Version 3.0 ed. Cancer Therapy Evaluation Program, Bethesda
Sonis S, Edwards L, Lucey C (1999) The biological basis for the attenuation of mucositis: the example of interleukin-11. Leukemia 13:831–834
Lockhart PB, Sonis ST (1981) Alterations in the oral mucosa caused by chemotherapeutic agents: a histologic study. J Dermatol Surg Oncol 7:1019–1025
Dorr W, Spekl K, Martin M (2002) Radiation-induced oral mucositis in mice: strain differences. Cell Prolif 35:60–67
Ulrich CM, Yasui Y, Storb R et al (2001) Pharmacogenetics of methotrexate: toxicity among marrow transplantation patients varies with the methylenetetrahydrofolate reductase C677T polymorphism. Blood 98:231–234
Hassan MG, Oberg AN, Bekassy J et al (1991) Pharmacokinetics of high-dose busulphan in relation to age and chronopharmacology. Cancer Chemother Pharmacol 28:130–134
Garming Legert K, Remberger M, Ringdén O, Hassan M, Dahllöf G (2011) Long-term salivary function after conditioning with busulfan, fractionated or single dose TBI. Oral Dis 17:670–676. doi:10.1111/j.1601-0825.2011.01821.x
Keefe D, Schubert M, Elting L et al (2007) Updated clinical practice guidelines for the prevention and treatment of mucositis. Cancer 109:820–831
Bhatt V, Vendrell N, Nau K, Crumb D, Roy V (2010) Implementation of a standardized protocol for prevention and management of oral mucositis in patients undergoing hematopoietic cell transplantation. J Oncol Pharm Pract 16:195–204
Katrancı N, Ovayolu N, Ovayolu O, Sevinc A (2012) Evaluation of the effect of cryotherapy in preventing oral mucositis associated with chemotherapy—a randomized controlled trial. Eur J Oncol Nurs 16:339–344
Sonis ST, Elting LS, Keefe D et al (2004) Perspectives on cancer therapy-induced mucosal injury: pathogenesis, measurement, epidemiology, and consequences for patients. Cancer 100:1995–2025
Barasch A, Peterson DE (2003) Risk factors for ulcerative oral mucositis in cancer patients: unanswered questions. Oral Oncol 39:91–100
Rapoport AP, Miller Watelet LF, Linder T et al (1999) Analysis of factors that correlate with mucositis in recipients of autologous and allogeneic stem-cell transplants. J Clin Oncol 17:2446–2453
Dreizen S (1990) Description and incidence of oral complications. NCI Monogr 9:11–15
Xiao C, Hanlon A, Zhang Q et al (2013) Symptom clusters in patients with head and neck cancer receiving concurrent chemoradiotherapy. Oral Oncol 49:360–366. doi:10.1016/j.oraloncology.2012.10.004. Epub 2012, PubMed PMID: 23168337
Acknowledgments
This work was supported by grants from the Swedish Cancer Society (0070-B06-20XBC), the Children’s Cancer Foundation (06/094), the Swedish Dental Society, the Swedish Research Council, and Karolinska Institutet. The authors thank dental hygienist Britta Tjärnberg Halvarsson for the competent oral care and assistance with OM scoring. The authors also thank the staff at the Center for Allogeneic Stem Cell Transplantation for the compassionate and competent care of the patients.
Conflict of interest
The authors declare that there are no conflicts of interest. The authors have full control of all primary data and agree to allow the journal to review their data if requested.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Legert, K.G., Remberger, M., Ringdén, O. et al. Reduced intensity conditioning and oral care measures prevent oral mucositis and reduces days of hospitalization in allogeneic stem cell transplantation recipients. Support Care Cancer 22, 2133–2140 (2014). https://doi.org/10.1007/s00520-014-2190-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-014-2190-7