Abstract
Purpose
Many cancer patients experience persistent fatigue after the completion of chemotherapy. A previous single-arm study provided evidence for an effect of acupuncture in this population. We conducted a randomized controlled trial to determine whether acupuncture reduces post-chemotherapy chronic fatigue more effectively than sham acupuncture.
Methods
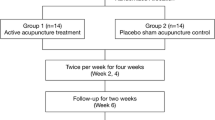
Cancer patients reporting significant fatigue persisting for at least 2 months following the completion of chemotherapy were randomized to receive once weekly true or sham acupuncture for 6 weeks. Fatigue was evaluated before and after treatment using the Brief Fatigue Inventory (BFI, the primary endpoint). Secondary endpoints included the Hospital Anxiety and Depression Scale (HADS) and Functional Assessment of Cancer Treatment-General (FACT-G) scores.
Results
One hundred one patients were randomized with 74 (34 true acupuncture; 40 sham control) evaluated for the primary endpoint. BFI scores fell by about one point between baseline and follow-up in both groups with no statistically significant difference between groups. HADS and FACT-G scores also improved in both groups, but there was no significant difference between groups. Patients in the sham acupuncture group crossed over to receive true acupuncture in week 7. No long-term reduction of fatigue scores was observed at the 6-month evaluation.
Conclusions
True acupuncture as provided in this study did not reduce post-chemotherapy chronic fatigue more than did sham acupuncture. The study is limited by the number of patients lost to follow-up. We also cannot exclude the possibility that a more intensive treatment regimen may be more effective.

Similar content being viewed by others
References
Aylard PR, Gooding JH, McKenna PJ, Snaith RP (1987) A validation study of three anxiety and depression self-assessment scales. J Psychosom Res 31:261–268
Balk J, Day R, Rosenzweig M, Beriwal S (2009) Pilot, randomized, modified, double-blind, placebo-controlled trial of acupuncture for cancer-related fatigue. J Soc Integr Oncol 7:4–11
Bower JE, Ganz PA, Desmond KA, Rowland JH, Meyerowitz BE, Belin TR (2000) Fatigue in breast cancer survivors: occurrence, correlates, and impact on quality of life. J Clin Oncol 18:743–753
Butt Z, Rosenbloom SK, Abernethy AP, Beaumont JL, Paul D, Hampton D, Jacobsen PB, Syrjala KL, Von Roenn JH, Cella D (2008) Fatigue is the most important symptom for advanced cancer patients who have had chemotherapy. J Natl Compr Canc Netw 6:448–455
Campos MP, Hassan BJ, Riechelmann R, Del Giglio A (2011) Cancer-related fatigue: a practical review. Ann Oncol 22:1273–1279
Cella DF, Tulsky DS, Gray G, Sarafian B, Linn E, Bonomi A, Silberman M, Yellen SB, Winicour P, Brannon J et al (1993) The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol 11:570–579
Goldstein D, Bennett BK, Webber K, Boyle F, de Souza PL, Wilcken NR, Scott EM, Toppler R, Murie P, O’Malley L, McCourt J, Friedlander M, Hickie IB, Lloyd AR (2012) Cancer-related fatigue in women with breast cancer: outcomes of a 5-year prospective cohort study. J Clin Oncol
Iop A, Manfredi AM, Bonura S (2004) Fatigue in cancer patients receiving chemotherapy: an analysis of published studies. Ann Oncol 15:712–720
Johnston MF, Hays RD, Subramanian SK, Elashoff RM, Axe EK, Li JJ, Kim I, Vargas RB, Lee J, Yang L, Hui KK (2011) Patient education integrated with acupuncture for relief of cancer-related fatigue randomized controlled feasibility study. BMC Complement Altern Med 11:49
Kaptchuk TJ, Stason WB, Davis RB, Legedza AR, Schnyer RN, Kerr CE, Stone DA, Nam BH, Kirsch I, Goldman RH (2006) Sham device v inert pill: randomised controlled trial of two placebo treatments. BMJ 332:391–397
Kleinhenz J, Streitberger K, Windeler J, Gussbacher A, Mavridis G, Martin E (1999) Randomised clinical trial comparing the effects of acupuncture and a newly designed placebo needle in rotator cuff tendinitis. Pain 83:235–241
Mao JJ, Styles T, Cheville A, Wolf J, Fernandes S, Farrar JT (2009) Acupuncture for nonpalliative radiation therapy-related fatigue: feasibility study. J Soc Integr Oncol 7:52–58
Meeske K, Smith AW, Alfano CM, McGregor BA, McTiernan A, Baumgartner KB, Malone KE, Reeve BB, Ballard-Barbash R, Bernstein L (2007) Fatigue in breast cancer survivors two to five years post diagnosis: a HEAL study report. Qual Life Res 16:947–960
Mendoza TR, Wang XS, Cleeland CS, Morrissey M, Johnson BA, Wendt JK, Huber SL (1999) The rapid assessment of fatigue severity in cancer patients: use of the Brief Fatigue Inventory. Cancer 85:1186–1196
Mock V, Atkinson A, Barsevick A, Cella D, Cimprich B, Cleeland C, Donnelly J, Eisenberger MA, Escalante C, Hinds P, Jacobsen PB, Kaldor P, Knight SJ, Peterman A, Piper BF, Rugo H, Sabbatini P, Stahl C (2000) NCCN Practice guidelines for cancer-related fatigue. Oncology (Williston Park) 14:151–161
Molassiotis A, Sylt P, Diggins H (2007) The management of cancer-related fatigue after chemotherapy with acupuncture and acupressure: a randomised controlled trial. Complement Ther Med 15:228–237
Streitberger K, Kleinhenz J (1998) Introducing a placebo needle into acupuncture research. Lancet 352:364–365
Vainio A, Auvinen A (1996) Prevalence of symptoms among patients with advanced cancer: an international collaborative study. Symptom Prevalence Group. J Pain Symptom Manage 12:3–10
Vickers AJ, Straus DJ, Fearon B, Cassileth BR (2004) Acupuncture for postchemotherapy fatigue: a phase II study. J Clin Oncol 22:1731–1735
Acknowledgments
The authors would like to thank Carrie Trevisan, Kristofer Prepelica, James Lozada, Marci Coleton, Gria Jacobs, Maria Kryza, and Ingrid Haviland for their assistance in the conduct of the study and the preparation of the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Deng, G., Chan, Y., Sjoberg, D. et al. Acupuncture for the treatment of post-chemotherapy chronic fatigue: a randomized, blinded, sham-controlled trial. Support Care Cancer 21, 1735–1741 (2013). https://doi.org/10.1007/s00520-013-1720-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-013-1720-z