Summary
Background
During transitions of care, patient’s medications are prone to medication errors. This study evaluated the impact of pharmacist-led medication reconciliation at hospital admission on unintentional medication discrepancies and adverse drug events.
Methods
A randomized controlled clinical trial was conducted in 120 adult medical patients hospitalized in a tertiary hospital in Slovenia. In the intervention group, a pharmacist-led medication reconciliation was performed on admission, while the control group received usual care. Patient’s drug treatment before admission was compared with their admission and inpatient treatment to identify discrepancies. The intention of discrepancies and related adverse drug events were assessed as a consensus of an expert panel.
Results
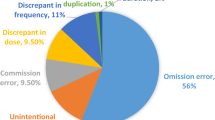
Included patients were elderly (median 72 years) and treated with polypharmacy (median 7 medications). Upon admission, discrepancies and unintentional discrepancies, representing a medication error, were identified in 61.2% (825/1347) and 18.3% (247/1347) of medications, respectively. In the intervention group, only 29.1% (37/127) of unintentional discrepancies were reported to the physicians in person. The majority of admission discrepancies (88%) persisted through hospitalization. Unintentional discrepancies resulted in 51 adverse drug events even during hospitalization. There were no differences between the intervention and control group in the occurrence of unintentional discrepancies (p = 0.481) or adverse drug events (p = 0.801).
Conclusions
Medication reconciliation at hospital admission failed to reduce unintentional discrepancies and adverse drug events, possibly due to its poor integration into clinical practice. Discrepancies resulted in patient harm even during the short period of hospitalization, which warrants the implementation of medication reconciliation at hospital admission.

Similar content being viewed by others
References
Tam VC. Frequency, type and clinical importance of medication history errors at admission to hospital: a systematic review. Can Med Assoc J. 2005;173(5):510–5.
Cornish PL, Knowles SR, Marchesano R, Tam V, Shadowitz S, Juurlink DN, et al. Unintended medication discrepancies at the time of hospital admission. Arch Intern Med. 2005;165(4):424.
Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients. JAMA. 1998;279(15):1200.
Grimes TC, Duggan CA, Delaney TP, Graham IM, Conlon KC, Deasy E, et al. Medication details documented on hospital discharge: cross-sectional observational study of factors associated with medication non-reconciliation. Br J Clin Pharmacol. 2011;71(3):449–57.
Bates DW. Incidence of adverse drug events and potential adverse drug events. JAMA. 1995;274(1):29.
de Vries EN, Ramrattan MA, Smorenburg SM, Gouma DJ, Boermeester MA. The incidence and nature of in-hospital adverse events: a systematic review. Qual Saf Health Care. 2008;17(3):216–23.
Jatau AI, Aung MMT, Kamauzaman THT, Rahman AFA. Prevalence of drug-related emergency department visits at a teaching hospital in Malaysia. Drugs Real World Outcomes. 2015;2(4):387–95.
Klopotowska JE, Wierenga PC, Stuijt CCM, Arisz L, Dijkgraaf MGW, Kuks PFM, et al. Adverse drug events in older hospitalized patients: results and reliability of a comprehensive and structured identification strategy. PLoS One. 2013;8(8):e71045.
Samoy LJ, Zed PJ, Wilbur K, Balen RM, Abu-Laban RB, Roberts M. Drug-related hospitalizations in a tertiary care internal medicine service of a Canadian hospital: a prospective study. Pharmacotherapy. 2006;26(11):1578–86.
von Laue NC, Schwappach DLB, Koeck CM. The epidemiology of preventable adverse drug events: a review of the literature. Wien Klin Wochenschr. 2003;115(12):407–15.
Kripalani S. Effect of a pharmacist intervention on clinically important medication errors after hospital discharge. Ann Intern Med. 2012;157(1):1.
Schnipper JL, Kirwin JL, Cotugno MC, Wahlstrom SA, Brown BA, Tarvin E, et al. Role of pharmacist counseling in preventing adverse drug events after hospitalization. Arch Intern Med. 2006;166(5):565–71.
International Pharmaceutical Federation. Medicines reconciliation: a toolkit for pharmacists. 2021[Online]. Available at: https://www.fip.org/file/4949. Accessed 17 July 2021.
The Joint Commission. National Patient Safety Goals® effective January 2021 for the hospital program. 2021 [Online]. Available at: https://www.jointcommission.org/-/media/tjc/documents/standards/national-patient-safety-goals/2021/npsg_chapter_hap_jan2021.pdf. Accessed 17 July 2021.
Ensing HT, Stuijt CCM, van den Bemt BJF, van Dooren AA, Karapinar-Çarkit F, Koster ES, et al. Identifying the optimal role for pharmacists in care transitions: a systematic review. J Manag Care Spec Pharm. 2015;21(8):614–36.
Cebron Lipovec N, Zerovnik S, Kos M. Pharmacy-supported interventions at transitions of care: an umbrella review. Int J Clin Pharm. 2019;41(4):831–52.
Michaelsen MH, McCague P, Bradley CP, Sahm LJ. Medication reconciliation at discharge from hospital: a systematic review of the quantitative literature. Pharmacy (Basel). 2015;3(2):53–71.
Redmond P, Grimes TC, McDonnell R, Boland F, Hughes C, Fahey T. Impact of medication reconciliation for improving transitions of care. Cochrane Database Syst Rev. 2018;8:CD10791.
Gillespie U, Alassaad A, Henrohn D, Garmo H, Hammarlund-Udenaes M, Toss H, et al. A comprehensive pharmacist intervention to reduce morbidity in patients 80 years or older. Arch Intern Med. 2009;169(9):894.
Kwan JL, Lo L, Sampson M, Shojania KG. Medication reconciliation during transitions of care as a patient safety strategy: a systematic review. Ann Intern Med. 2013;158(5 Pt 2):397–403.
Knez L, Suskovic S, Rezonja R, Laaksonen R, Mrhar A. The need for medication reconciliation: a cross-sectional observational study in adult patients. Respir Med. 2011;105:S60–S6.
Režonja R, Knez L, Šuškovič S, Košnik M, Mrhar A. Comprehensive medication history: the need for the implementation of medication reconciliation processes. Slov J Public Health. 2010;49(4):202–10.
Morimoto T. Adverse drug events and medication errors: detection and classification methods. Qual Saf Health Care. 2004;13(4):306–14.
Van den Bemt P, Egberts A. Drug-related problems: definitions and classification. EJHP Pract. 2007;13:62–4.
Bates DW, Leape LL, Petrycki S. Incidence and preventability of adverse drug events in hospitalized adults. J Gen Intern Med. 1993;8(6):289–94.
Vira T, Colquhoun MEE. Reconcilable differences: correcting medication errors at hospital admission and discharge. Qual Saf Health Care. 2006;15:122–6.
Acknowledgements
The authors would like to thank Anja Primožič and Sara Cimprič for their contribution to data collection, Prof. Mitja Košnik and Prof. Stanislav Šuškovič for their participation in the expert panel, and all participating pharmacists.
Funding
The authors acknowledge the financial support from the Slovenian Research Agency (research core funding No. P1-0189 and P‑0360).
Author information
Authors and Affiliations
Contributions
The study was conceptualized and designed by Lea Knez and Aleš Mrhar. Material preparation, data collection and analysis were performed by Lea Knez, Maja Jošt and Mojca Kerec Kos. The first draft of the manuscript was written by Maja Jošt and all other authors Lea Knez, Aleš Mrhar, Mojca Kerec Kos critically revised the manuscript for the important intellectual content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
M. Jošt, L. Knez, A. Mrhar and M.K. Kos declare that they have no competing interests.
Ethical standards
All procedures performed in the study were in accordance with the ethical standards of the institutional and/or national research committee (National Medical Ethics Committee in Slovenia (Protocol Number 0120-223/2019/4)) and with the 1975 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jošt, M., Knez, L., Mrhar, A. et al. Adverse drug events during transitions of care. Wien Klin Wochenschr 134, 130–138 (2022). https://doi.org/10.1007/s00508-021-01972-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00508-021-01972-2