Summary
Aims
We aimed to determine the contribution of quantitative HBsAg in differentiating chronic infections from chronic hepatitis in HBeAg negative patients with HBV DNA 2000–20,000 IU/ml.
Material and methods
A total of 79 untreated HBeAg negative patients were included. Patients were divided into 3 groups based on HBV DNA levels: group 1 (HBV DNA ≤ 2000 IU/ml), group 2 (HBV DNA: 2000–20,000 IU/ml) and group 3 (HBV DNA > 20,000 IU/ml). We collected serum from all patients for quantitative HBsAg analysis. We compared serum quantitative HBsAg levels with biochemical parameters, HBV DNA and liver biopsy results.
Results
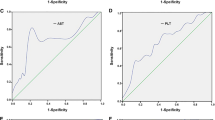
In this study 46 patients were female and the mean age was 42 years. Serum quantitative HBsAg levels were found to be significantly lower in chronic infections compared with chronic hepatitis. There was a positive correlation between quantitative HBsAg and HBV DNA, ALT (alanine aminotransferase), HAI score (histological activity index), fibrosis score and disease stage. The cut-off level of quantitative HBsAg was determined as 4425 IU/ml to differentiate chronic infection from chronic hepatitis. With the test specificity of 95%, we found quantitative HBsAg cut-off values 1026 IU/ml and 20,346 IU/ml for the diagnosis of chronic infection and chronic hepatitis, respectively.
Conclusion
Our study suggests that the quantitative HBsAg ≤ 1000 IU/ml limit value might be used for the diagnosis of chronic infection not only in HBV DNA ≤ 2000 IU/ml but also in patients with HBV DNA between 2000–20,000 IU/ml. In addition, antiviral treatment could be considered in patients with quantitative HBsAg > 20,000 IU/ml and HBV DNA > 2000 IU/ml without further examinations such as liver biopsy.




Similar content being viewed by others
References
Seeger C, Mason WS. Hepatitis B virus biology. Microbiol Mol Biol Rev. 2000;64:51–68.
Liang TJ. Hepatitis B : the virus and disease. 2009. pp. 13–21.
Gerlich WH. Medical Virology of Hepatitis B: how it began and where we are now. Virol J. 2013;10:239.
Tseng TC, Kao JH. Clinical utility of quantitative HBsAg in natural history and nucleos(t)ide analogue treatment of chronic hepatitis B: New trick of old dog. J Gastroenterol. 2013;48:13–21.
Janssen HL, Kerhof-Los CJ, Heijtink RA, Schalm SW. Measurement of HBsAg to monitor hepatitis B viral replication in patients on alpha-interferon therapy. Antiviral Res. 1994;23:251–7.
Brunetto MR, Oliveri F, Colombatto P, Moriconi F, Ciccorossi P, Coco B, et al. Hepatitis B surface antigen serum levels help to distinguish active from inactive hepatitis B virus genotype D carriers. Gastroenterology. 2010;139:483–90.
Lampertico P, Agarwal K, Berg T, Buti M, Janssen HLA, Papatheodoridis G, et al. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370–98.
Zoulim F, Carosi G, Greenbloom S, Mazur W, Nguyen T, Jeffers L, et al. Quantification of HBsAg in nucleos ( t ) ide-naïve patients treated for chronic hepatitis B with entecavir with or without tenofovir in the BE-LOW study. J Hepatol. 2015;62:56–63.
Fasano M, Lampertico P, Marzano A, Di Marco V, Niro GA, Brancaccio G, et al. HBV DNA suppression and HBsAg clearance in HBeAg negative chronic hepatitis B patients on lamivudine therapy for over 5 years. J Hepatol. 2012;56:1254–8.
Brunetto MR, Marcellin P, Cherubini B, Yurdaydin C, Farci P, Hadziyannis SJ, et al. Response to peginterferon alfa-2a ( 40KD ) in HBeAg-negative CHB : On-treatment kinetics of HBsAg serum levels vary by HBV genotype. J Hepatol. 2013;59:1153–9.
Seto W, Wong DK, Fung J, Huang F, Liu KS, Lai C, et al. Linearized hepatitis B surface antigen and hepatitis B core-related antigen in the natural history of chronic hepatitis B. Eur Soc Clin Infect Dis. 2014;20:1173–80.
Brunetto MR, Moriconi F, Bonino F. Hepatitis B virus surface antigen levels: A guide to sustained response to peginterferon alfa-2a in HBeAg-negative chronic hepatitis B. Hepatology. 2009;49:1141–50.
Larsson SB, Eilard A, Malmström S, Hannoun C, Dhillon AP, Norkrans G, et al. HBsAg quantification for identification of liver disease in chronic hepatitis B virus carriers. Liver Int. 2013;34:e238–45. https://doi.org/10.1111/liv.12345.
Wisedopas N, Poovorawan Y, Tangkijvanich P. Kinetics of serum HBsAg and Intrahepatic cccDNA during Pegylated interferon terapy in patients with HbeAg-positive and HbeAg-negative chronic hepatitis B. J Med Virol. 2017;89:130–8.
Bréchot C. Pathogenesis of hepatitis B virus-related hepatocellular carcinoma: old and new paradigms. Gastroenterology. 2004;127:S56–S61.
Höner zu Siederdissen C, Maasoumy B, Cornberg M. What is new on HBsAg and other diagnostic markers in HBV infection? Best Pract Res Clin Gastroenterol. 2014;31:281–9.
Association E. EASL Clinical Practice Guidelines : management of chronic hepatitis B virus infection. J Hepatol. 2012;57:167–85.
Terrault NA, Bzowej NH, Chang K‑M, Hwang JP, Jonas MM, Murad MH. AASLD guidelines for treatment of chronic hepatitis B. Hepatology. 2016;63:261–83.
Sarin SK, Kumar M, Lau GK, Abbas Z, Chan HLY, Chen CJ, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. 2016;10:1–98.
Iloeje U, Yang H, Su J, Jen C, You S, Chen C. Predicting cirrhosis risk based on the level of circulating hepatitis B viral load. Gastroenterology. 2006;130:678–86.
Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, et al. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696–9.
Larsson SB, Hannoun C, Lindh M, Malmstro S. Hepatitis B viral DNA decline at loss of HbeAg is mainly explained by reduced cccDNA load—down-regulated transcription of PgRNA has limited impact. Plos One. 2012;7:e36349.
Weng M, Zeng W, Wu X, Zhang Y, Jiang M, Wang Z, et al. Quantification of serum hepatitis B surface antigen in predicting the response of pegylated interferon alfa-2a in HBeAg-positive chronic hepatitis B with prior lamivudine exposure. Virol J. 2013;10:1.
Janssen HLA, Sonneveld MJ, Brunetto MR. Quantification of serum hepatitis B surface antigen : is it useful for the management of chronic hepatitis B ? Gut. 2012;61:641–5.
Chan HL, Thompson A, Martinot-peignoux M, Piratvisuth T, Cornberg M, Brunetto MR, et al. Review hepatitis B surface antigen quantification : why and how to use it in 2011—A core group report. J Hepatol. 2011;55:1121–31.
Alghamdi A, Aref N, El-Hazmi M, Al-Hamoudi W, Alswat K, Helmy A, et al. Correlation between hepatitis B surface antigen titers and HBV DNA levels. Saudi J Gastroenterol. 2013;19:252–7.
Sali S, Sharafi H, Hoda S, Moayed S, Etesam F. Can serum level of HBsAg differentiate HBeAg-negative chronic hepatitis B from inactive carrier state ? Diagn Microbiol Infect Dis. 2015;82:114–9.
Balkan A, Namıduru M, Balkan Y, Mete AÖ, Karaoğlan İ. Are serum quantitative hepatitis B surface antigen levels , liver histopathology and viral loads related in chronic hepatitis B—infected patients ? Saudi J Gastroenterol. 2016;22:208–14.
Nguyen T, Thompson AJV, Bowden S, Croagh C, Bell S, Desmond PV, et al. Hepatitis B surface antigen levels during the natural history of chronic hepatitis B : a perspective on Asia. J Hepatol. 2010;52:508–13.
Thompson AJV, Nguyen T, Iser D, Ayres A, Jackson K, Littlejohn M, et al. Serum hepatitis B surface antigen and hepatitis B e antigen titers: disease phase influences correlation with viral load and intrahepatic hepatitis B virus markers. Hepatology. 2010;51:1933–44. https://doi.org/10.1002/hep.23571.
Michelle M‑P, Lapalus M, Laouénan C, Boyer N, -Pierre Ripault M, Asselah T, et al. How to distinguish HBeAg negative chronic hepatitis B, with high risk of reactivation, from inactive carriers: Is there a place for HBsAg quantification? Hepatology. 2012;56:434A–5A.
Tseng T, Liu C, Yang H, Su T, Wang C, Chen C, et al. High levels of hepatitis B surface antigen increase risk of hepatocellular carcinoma in patients with low HBV load. Gastroenterology. 2012;142:1140–9.
Funding
This study was supported financially by Istanbul University Scientific Research Projects Coordination Unit (project code: TTU-2017-24508).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
S. Yıldız Kaya, B. Mete, A. Kaya, I. I. Balkan, N. Saltoglu and Ö. F. Tabak declare that they have no competing interests.
Ethical standards
All procedures performed in studies involving human participants or on human tissue were in accordance with the ethical standards of the ethical committee of Istanbul University Cerrahpasa Medical Faculty (83045809-604.01.02)/or national research committee and with the 1975 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
All authors meet the International Committee of Medical Journal Editors (ICMJE) authorship criteria.
Rights and permissions
About this article
Cite this article
Yıldız Kaya, S., Mete, B., Kaya, A. et al. The role of quantitative HBsAg in patients with HBV DNA between 2000–20,000 IU/ml. Wien Klin Wochenschr 133, 647–653 (2021). https://doi.org/10.1007/s00508-021-01854-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00508-021-01854-7