Abstract
Background
Regional citrate anticoagulation (RCA) is the preferred continuous kidney replacement therapy (CKRT) anticoagulation strategy for children in the USA. Nafamostat mesilate (NM), a synthetic serine protease, is used widely for CKRT anticoagulation in Japan and Korea. We compared the safety and efficacy of NM to RCA for pediatric CKRT.
Methods
Starting June 2019, the most recent 100 medical records of children receiving CKRT with either RCA or NM were reviewed retrospectively, at one children’s hospital in Japan (NM) and one in the USA (RCA). The number of hours a single CKRT filter was in use, was the primary outcome. Safety was assessed by bleeding complications for the NM group and citrate toxicity leading to RCA discontinuation or electrolyte imbalance in the RCA group.
Results
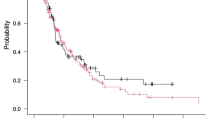
Eighty patients received NM and 78 patients received RCA. Median filter life was longer for the NM group (NM: 38 [22, 74] vs. RCA: 36 [17, 66] h, p = 0.02). When filter life was censored for discontinuation other than clotting, the 60-h survival rate was higher for RCA (71% vs. 54%). The hazard ratio comparing NM over RCA varied over time (HR 0.7; 0.2–1.5, p = 0.33 at 0 h to HR 5.5; 1.3–23.7, p = 0.334 at 72 h). The lack of difference in filter survival persisted controlling for filter surface area, catheter diameter, and pre-CKRT platelet count. Major bleeding rates did not differ between groups (NM: 5% vs. RCA: 9%).
Conclusions
RCA and NM provide satisfactory anticoagulation for CKRT in children with no difference in major bleeding rates.
Graphical abstract
A higher resolution version of the Graphical abstract is available as Supplementary information.



Similar content being viewed by others
References
(2012) Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group - KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int suppl 2:1–138
Rodriguez K, Srivaths PR, Tal L, Watson MN, Riley AA, Himes RW, Desai MS, Braun MC, Akcan Arikan A (2017) Regional citrate anticoagulation for continuous renal replacement therapy in pediatric patients with liver failure. PLoS One 12:e0182134
Schultheiss C, Saugel B, Phillip V, Thies P, Noe S, Mayr U, Haller B, Einwachter H, Schmid RM, Huber W (2012) Continuous venovenous hemodialysis with regional citrate anticoagulation in patients with liver failure: a prospective observational study. Crit Care 16:R162
Zhang W, Bai M, Yu Y, Li L, Zhao L, Sun S, Chen X (2019) Safety and efficacy of regional citrate anticoagulation for continuous renal replacement therapy in liver failure patients: a systematic review and meta-analysis. Crit Care 23:22
Klingele M, Stadler T, Fliser D, Speer T, Groesdonk HV, Raddatz A (2017) Long-term continuous renal replacement therapy and anticoagulation with citrate in critically ill patients with severe liver dysfunction. Crit Care 21:294
Tan JN, Haroon SWP, Mukhopadhyay A, Lau T, Murali TM, Phua J, Tan ZY, Lee N, Chua HR (2019) Hyperlactatemia predicts citrate intolerance with regional citrate anticoagulation during continuous renal replacement therapy. J Intensive Care Med 34:418–425
Oudemans-van Straaten HM, Ostermann M (2012) Bench-to-bedside review: Citrate for continuous renal replacement therapy, from science to practice. Crit Care 16:249
Chen X, Xu Z, Zeng S, Wang X, Liu W, Qian L, Wei J, Yang X, Shen Q, Gong Z, Yan Y (2019) The molecular aspect of antitumor effects of protease inhibitor nafamostat mesylate and its role in potential clinical applications. Front Oncol 9:852
Yang JW, Han BG, Kim BR, Lee YH, Kim YS, Yu JM, Choi SO (2009) Superior outcome of nafamostat mesilate as an anticoagulant in patients undergoing maintenance hemodialysis with intracerebral hemorrhage. Ren Fail 31:668–675
Fuse I, Higuchi W, Toba K, Aizawa Y (1999) Inhibitory mechanism of human platelet aggregation by nafamostat mesilate. Platelets 10:212–218
Maruyama Y, Yoshida H, Uchino S, Yokoyama K, Yamamoto H, Takinami M, Hosoya T (2011) Nafamostat mesilate as an anticoagulant during continuous veno-venous hemodialysis: a three-year retrospective cohort study. Int J Artif Organs 34:571–576
Hu ZJ, Iwama H, Suzuki R, Kobayashi S, Akutsu I (1999) Time course of activated coagulation time at various sites during continuous haemodiafiltration using nafamostat mesilate. Intensive Care Med 25:524–527
Lee YK, Lee HW, Choi KH, Kim BS (2014) Ability of nafamostat mesilate to prolong filter patency during continuous renal replacement therapy in patients at high risk of bleeding: a randomized controlled study. PLoS One 9:e108737
Choi JY, Kang YJ, Jang HM, Jung HY, Cho JH, Park SH, Kim YL, Kim CD (2015) Nafamostat mesilate as an anticoagulant during continuous renal replacement therapy in patients with high bleeding risk: a randomized clinical trial. Medicine 94:e2392
Baek NN, Jang HR, Huh W, Kim YG, Kim DJ, Oh HY, Lee JE (2012) The role of nafamostat mesylate in continuous renal replacement therapy among patients at high risk of bleeding. Ren Fail 34:279–285
Hwang SD, Hyun YK, Moon SJ, Lee SC, Yoon SY (2013) Nafamostat mesilate for anticoagulation in continuous renal replacement therapy. Int J Artif Organs 36:208–216
Makino S, Egi M, Kita H, Miyatake Y, Kubota K, Mizobuchi S (2016) Comparison of nafamostat mesilate and unfractionated heparin as anticoagulants during continuous renal replacement therapy. Int J Artif Organs 39:16–21
Lee SC, Cho H (2014) The use of nafamostat mesilate as an anticoagulant during continuous renal replacement therapy for children with a high risk of bleeding. J Korean Soc Pediatr Nephrol 18:98–105
Slater A, Shann F, Pearson G, Paediatric Index of Mortality Study Group (2003) PIM2: a revised version of the Paediatric Index of Mortality. Intensive Care Med 29:278–285
Goldstein SL, Askenazi DJ, Basu RK, Selewski DT, Paden ML, Krallman KA, Kirby CL, Mottes TA, Terrell T, Humes HD (2021) Use of the selective cytopheretic device in critically ill children. Kidney Int Rep 6:775–784
Sampson HA, Munoz-Furlong A, Campbell RL, Adkinson NF Jr, Bock SA, Branum A, Brown SG, Camargo CA Jr, Cydulka R, Galli SJ, Gidudu J, Gruchalla RS, Harlor AD Jr, Hepner DL, Lewis LM, Lieberman PL, Metcalfe DD, O’Connor R, Muraro A, Rudman A, Schmitt C, Scherrer D, Simons FE, Thomas S, Wood JP, Decker WW (2006) Second symposium on the definition and management of anaphylaxis: summary report–Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. J Allergy Clin Immunol 117:391–397
Brophy PD, Somers MJ, Baum MA, Symons JM, McAfee N, Fortenberry JD, Rogers K, Barnett J, Blowey D, Baker C, Bunchman TE, Goldstein SL (2005) Multi-centre evaluation of anticoagulation in patients receiving continuous renal replacement therapy (CRRT). Nephrol Dial Transplant 20:1416–1421
Bai M, Zhou M, He L, Ma F, Li Y, Yu Y, Wang P, Li L, Jing R, Zhao L, Sun S (2015) Citrate versus heparin anticoagulation for continuous renal replacement therapy: an updated meta-analysis of RCTs. Intensive Care Med 41:2098–2110
Raymakers-Janssen P, Lilien M, van Kessel IA, Veldhoen ES, Wosten-van Asperen RM, van Gestel JPJ (2017) Citrate versus heparin anticoagulation in continuous renal replacement therapy in small children. Pediatr Nephrol 32:1971–1978
Brain M, Winson E, Roodenburg O, McNeil J (2017) Non anti-coagulant factors associated with filter life in continuous renal replacement therapy (CRRT): a systematic review and meta-analysis. BMC Nephrol 18:69
Uchino S, Fealy N, Baldwin I, Morimatsu H, Bellomo R (2003) Pre-dilution vs. post-dilution during continuous veno-venous hemofiltration: impact on filter life and azotemic control. Nephron Clin Pract 94:c94-98
van der Voort PH, Gerritsen RT, Kuiper MA, Egbers PH, Kingma WP, Boerma EC (2005) Filter run time in CVVH: pre- versus post-dilution and nadroparin versus regional heparin-protamine anticoagulation. Blood Purif 23:175–180
Hackbarth R, Bunchman TE, Chua AN, Somers MJ, Baum M, Symons JM, Brophy PD, Blowey D, Fortenberry JD, Chand D, Flores FX, Alexander SR, Mahan JD, McBryde KD, Benfield MR, Goldstein SL (2007) The effect of vascular access location and size on circuit survival in pediatric continuous renal replacement therapy: a report from the PPCRRT registry. Int J Artif Organs 30:1116–1121
Dunn WJ, Sriram S (2014) Filter lifespan in critically ill adults receiving continuous renal replacement therapy: the effect of patient and treatment-related variables. Crit Care Resusc 16:225–231
Miklaszewska M, Korohoda P, Zachwieja K, Kobylarz K, Stefanidis CJ, Sobczak A, Drozdz D (2017) Filter size not the anticoagulation method is the decisive factor in continuous renal replacement therapy circuit survival. Kidney Blood Press Res 42:327–337
Han SJ, Han W, Song HJ, Kim CS, Jeong SM, Kang MW (2018) Validation of nafamostat mesilate as an anticoagulant in extracorporeal membrane oxygenation: a large-animal experiment. Korean J Thorac Cardiovasc Surg 51:114–121
Park JH, Her C, Min HK, Kim DK, Park SH, Jang HJ (2015) Nafamostat mesilate as a regional anticoagulant in patients with bleeding complications during extracorporeal membrane oxygenation. Int J Artif Organs 38:595–599
Lim JY, Kim JB, Choo SJ, Chung CH, Lee JW, Jung SH (2016) Anticoagulation during extracorporeal membrane oxygenation; nafamostat mesilate versus heparin. Ann Thorac Surg 102:534–539
Murase M, Usui A, Tomita Y, Maeda M, Koyama T, Abe T (1993) Nafamostat mesilate reduces blood loss during open heart surgery. Circulation 88:II432–436
Sawada K, Ohdo M, Ino T, Nakamura T, Numata T, Shibata H, Sakou J, Kusada M, Hibi T (2016) Safety and tolerability of nafamostat mesilate and heparin as anticoagulants in leukocytapheresis for ulcerative colitis: post hoc analysis of a large-scale, prospective, observational study. Ther Apher Dial 20:197–204
Miyatake Y, Makino S, Kubota K, Egi M, Mizobuchi S (2017) Association between intra-circuit activated clotting time and incidence of bleeding complications during continuous renal replacement therapy using nafamostat mesilate: a retrospective pilot observational study. Kobe J Med Sci 63:E30–E36
Acknowledgements
We thank Dr. Heather Baer (Department of Epidemiology, Harvard T.H. Chan School of Public Health), Dr. John Orav (Department of Biostatistics, Harvard T.H. Chan School of Public Health) and Dr. Takahiro Kinoshita (Philips Research North America) for methodological advice.
Author information
Authors and Affiliations
Contributions
MJM designed the study, analyzed the data, and drafted the paper; KI and KAK contributed to IRB preparation and subject screening; MM and KT contributed data collection; SLG guided the study and critically revised the paper; all authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
MJM serves as a consultant to Lowell Therapeutics, Inc, which has licensed NM in the US. At the current time, NM is not approved for marketing in the US. Lowell did not have input into the design of this study, access to the raw data or development of any aspect of this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Miyaji, M.J., Ide, K., Takashima, K. et al. Comparison of nafamostat mesilate to citrate anticoagulation in pediatric continuous kidney replacement therapy. Pediatr Nephrol 37, 2733–2742 (2022). https://doi.org/10.1007/s00467-022-05502-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-022-05502-8