Abstract
Background
AKI is an important complication post cardiac surgery in children. An early diagnosis can help in mitigating complications and allow for prognostication. Urinary albumin:creatinine ratio (ACR) as a biomarker can provide a cheaper and more accessible AKI risk assessment and prediction. There is a paucity of paediatric literature regarding its utility.
Methods
This was a prospective observational study, enrolling all children aged 1 month to 18 years, who underwent cardiac surgery, with use of cardiopulmonary bypass. Cohort was divided into groups < 2 years and ≥ 2 years for analyses to account for differences in physiological albumin excretion with age.
Results
Of 143 children enrolled in the study, 36 developed AKI. In both age groups, the post-operative ACR was higher than pre-operative ACR among patients with and without AKI. In the group aged ≥ 2 years, the highest first post-operative ACR tertile (> 75.8 mg/g) predicted post-operative AKI after adjusting for clinical variables (adjusted RR, 11.71; 1.85–16.59). In the group aged < 2 years, the highest first post-operative ACR tertile (> 141.3 mg/g) predicted post-operative AKI in unadjusted analysis but not after adjusting for clinical variables (RR, 2.78; 0.70–6.65). For AKI risk prediction, AUC (95% CI) was highest after combining clinical model and pre-operative ACR for groups aged < 2 years [0.805 (0.713-0.896)] and ≥ 2 years [0.872 (0.772–0.973)].
Conclusions
This study provides evidence for use of albuminuria as a feasible biomarker in AKI prediction in children post cardiac surgery, especially when added to a clinical model.
Graphical abstract
A higher resolution version of the Graphical abstract is available as Supplementary information.

Similar content being viewed by others
Introduction
Acute kidney injury (AKI) in children following cardiac surgery is common and associated with poor outcomes; it leads to increase in hospital stay, need for ventilator support, escalation of costs and even mortality [1,2,3,4]. The rise of serum creatinine is widely used for defining AKI; however, it often rises after significant kidney function has already been lost [5]. Biomarkers have shown utility with regard to a more expeditious diagnosis of AKI and prognostication of AKI as well as application of preventive strategies [5]. As previously studied, the use of biomarkers helped in enhancing the effectiveness of renal angina index (RAI) in AKI risk stratification of critically ill patients [6]. Similarly, a randomised trial in cardiac surgery patients was able to significantly reduce AKI by applying a care bundle in patients at high risk of kidney dysfunction, identified by the use of urinary biomarkers [7]. However, the applicability of most serum or urinary biomarkers is limited due to issues of cost, standardisation and lack of generalizability and being not yet in mainstream use. Urinary biomarker albumin:creatinine ratio (ACR) on the other hand, which is frequently used in assessment of chronic kidney disease, could provide a cheaper, more familiar and accessible option for AKI assessment [8].
As normal glomerular function limits albumin filtration and renal tubules are responsible for albumin reclamation, albuminuria can thus develop in AKI following ischemic or inflammatory insults to these structures [9, 10]. Various animal and human clinical studies have identified albuminuria developing in AKI states [9,10,11]. Studies in adults have identified pre-existing proteinuria as a risk factor for AKI in cardiac surgery [12, 13]. There have been limited studies in the paediatric population assessing albuminuria in AKI [14]. This study was designed to assess pre- and post-operative albuminuria in children undergoing cardiac surgery with cardiopulmonary bypass and its ability to predict AKI as described by KDIGO criteria [15].
Methods
Aim
To assess albuminuria as a predictive marker for AKI in children (1 month–18 years) undergoing cardiac surgery using cardiopulmonary bypass (open cardiac surgery) in both pre- and post-operative periods.
Study design
This was a prospective, observational study. Children undergoing cardiac surgery with use of cardiopulmonary bypass at our Paediatric Cardiothoracic unit from October 2017 to December 2018 were enrolled in this study.
Inclusion criteria
Children between 1 month and 18 years undergoing elective cardiac surgery were included.
Exclusion criteria
Children with pre-existing kidney dysfunction (baseline eGFR < 60 mL/min/1.73 m2, structural kidney abnormalities) or post kidney transplant were excluded, as well as those with incomplete data.
Informed consent was taken from all parents prior to the inclusion in the study and assent where applicable. This study was approved by the Research Ethics Board of the institution.
Collection of data
All children included in the study were admitted for open cardiac surgery at our cardiothoracic unit. Demographic variables including age, gender, weight and length were recorded prior to operative procedure. Data pertaining to diagnosis of heart disease, acyanotic/cyanotic heart disease and prior surgeries was collected. Glomerular filtration rate was estimated using the revised bedside Schwartz formula [16]. Urine ACR was measured in the pre-operative period. Urine was collected via Foley’s catheter and using a clean catch sample in those without urinary catheter.
Intraoperative variables such as duration of procedure, length of cardiopulmonary bypass and aortic cross clamp times were recorded. RACHS-1 category was assigned to each child; it is a consensus-based tool which defines short-term mortality risk in children undergoing surgery for congenital heart disease based on the type of procedure performed [17]. Post-operatively serum creatinine was measured daily until ICU stay and 1 day prior to hospital discharge. AKI was defined as per KDIGO definitions [15]. AKI stage 1 was defined as increase of serum creatinine ≥ 0.3 mg/dL within 48 h or ≥ 1.5- to two-fold from baseline or urine output ≤ 0.5 mL/kg/h for 6–12 h. AKI stage 2 was defined as a rise of serum creatinine 2.0–2.9 times from baseline or urine output ≤ 0.5 mL/kg/h for 12 h. AKI stage 3 was defined as a rise of serum creatinine 3.0 times from baseline or a rise to ≥ 4.0 mg/dL or initiation of kidney replacement therapy or, in patients < 18 years, decrease in eGFR to < 35 mL/min per 1.73 m2 or urine output ≤ 0.3 mL/kg/h for 24 h or anuria for 12 h [15].
Daily and cumulative fluid balances were recorded, as per the method used by Goldstein et al. [18]. Urine ACR was measured 6 h post-operatively on day 0, followed by daily for the next 2 days. Data pertaining to duration of mechanical ventilator support, use of NSAIDs/nephrotoxic drugs, duration of hospital stay, development of sepsis, transaminitis (more than two times the upper limit of normal), in-hospital mortality and development of low output cardiac syndrome (symptoms and signs of poor systemic perfusion such as tachycardia, cold extremities or development of acidosis such as increase in base deficit > 4 or increase in lactate by > 2 mg/dL in consecutive blood gas analysis; criteria used by Hoffman et al. [19]) were recorded. Haematological complications referred to development of anaemia and/or thrombocytopenia during admission. Diuretics and kidney replacement therapy were provided where required. Urine ACR was measured by an immunoturbidimetry method using the Architect ci-4100 analyser. Urine creatinine was measured by enzymatic method (creatinine amidohydrolase). All the analyses were conducted separately for children aged < 2 years and ≥ 2 years, as younger children tend to have physiological higher urinary albumin excretion which stabilises with tubular function maturation [14]. For the same reason, due to the variability in protein and creatinine excretion among neonates, especially early after birth, it was considered to exclude this age group from the analysis, as the physiological variations may become a confounding factor in evaluation.
Statistical analyses
The variables were recorded in Microsoft Excel and statistical software (SPSS version 22) was used for the statistical analyses. All the variables were tested for normality using the Kolmogorov–Smirnov test. The outcomes include AKI incidence (yes vs. no, and stage ≥ 2 vs. stage 1), length of stay (ICU and hospital) and mortality. The categorical variables were summarized as frequencies and percentages, while continuous variables as median and interquartile range (IQR; 25th to 75th percentiles) for the sake of consistency. The association between categorical variables was assessed using the chi-square statistic or Fischer exact test, while continuous variables with the Mann–Whitney U test or Kruskal–Wallis test. Generalized linear model was used to calculate the unadjusted and adjusted relative risks (RRs) of pre-operative and post-operative day 0 (first post-operative) ACR for the prediction of AKI. The pre-operative ACR was categorized as ACR < 10 mg/g, ACR 10–30 mg/g and ACR > 30 mg/g, while the first post-operative ACR was categorized based on its tertile values.
The multivariate clinical model comprised multiple predictors, such as age (continuous in month for the group aged < 2 years; categorical for the group aged ≥ 2 years: 2 – < 6 vs. ≥ 6 years); gender (categorical: male vs. female); previous cardiac surgeries (categorical: yes vs. no); type of heart surgery (categorical: cyanotic vs. acyanotic); RACHS-1 score (categorical: ≥ 3 vs. < 3); cardiopulmonary bypass time (categorical: < 120 vs. ≥ 120 min); pre-operative creatinine (continuous in mg/dL) and pre-operative estimated glomerular filtration rate (eGFR; continuous in mL/min/1.73 m2).
The different diagnostic characteristics (sensitivity, specificity, positive predictive value and negative predictive value) of pre-operative and first post-operative ACR threshold values for AKI prediction were conducted. The area under the receiver operating characteristic curve (AUC; with 95% confidence interval (95%CI)) was calculated to determine the ability of clinical model alone, pre-operative ACR alone, first post-operative ACR alone and combinations of these to discriminate between patients with and without AKI. A two tailed p value < 0.05 was considered to be statistically significant.
Results
Patient characteristics
The study flow is shown in Supplementary Fig. 1. In the group aged < 2 years, the median (IQR) age was significantly lower among those with higher ACR level (ACR < 10 mg/g: 12 (-) months vs. ACR 10–30 mg/g: 9 (7.3–12) months vs. ACR > 30 mg/g: 6 (4–12) months; p < 0.05) and the proportion of AKI incidence was significantly higher among those with higher ACR level (ACR < 10 mg/g: 0% vs. ACR 10–30 mg/g: 5% vs. ACR > 30 mg/g: 35%; p < 0.05). All other variables did not differ significantly across the three pre-operative ACR levels. Similarly, among the group aged ≥ 2 years, none of the variables differed significantly across the three pre-operative ACR levels (Table 1). Out of 143 children, 93 (65%) were aged < 2 years and 50 (35%) were aged ≥ 2 years. Thirty-six children developed AKI; 26 were < 2 years and 10 were ≥ 2 years of age. Four children required peritoneal dialysis. AKI occurred first on mean POD 1.96 (SD 1.34) in children < 2 years and on mean POD 1.5 in children ≥ 2 years (SD 0.97).
Association of pre-operative and post-operative ACR with AKI
In both age groups, the post-operative ACR was much higher than pre-operative ACR among patients with and without AKI. A fall in urine ACR was also seen in all age groups by POD2. In the group aged < 2 years, the median (IQR) ACR was significantly higher among those with AKI as compared to those without AKI: at pre-operative (64.5 (41–86.8) vs. 40 (25–55) mg/g; p < 0.05), at post-operative day 0 (153 (98–347.8) vs. 99 (54–142) mg/g; p < 0.05), at post-operative day 1 (283.5 (127–455) vs. 97 (68–164) mg/g; p < 0.001) and at post-operative day 2 (197.5 (89–355) vs. 74 (53–122) mg/g; p < 0.001). However, in the group aged ≥ 2 years, the median (IQR) was significantly higher among those with AKI as compared to those without AKI only at post-operative day 0 (131.5 (78–362.5) vs. 48 (22–75) mg/g; p < 0.05) (Table 2).
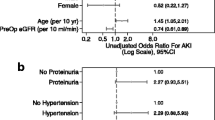
In the group aged < 2 years, the pre-operative ACR > 30 mg/g predicted post-operative AKI in unadjusted analysis (RR 7.29; 95% CI, 1.05–50.68) but not after adjusting for clinical variables (RR 4.91; 0.7–15.33) (Table 3). In the group aged ≥ 2 years, the pre-operative ACR > 30 mg/g was not significantly associated with post-operative AKI. Also, in the group aged < 2 years, pre-operative ACR > 30 mg/g was not significantly associated with the development of stage ≥ 2 AKI. Meanwhile, in the group aged ≥ 2 years, it was not possible to calculate the risk for pre-operative ACR to predict stage ≥ 2 AKI due to low event numbers (Table 3).
In the group aged ≥ 2 years, the highest first post-operative ACR tertile (> 75.8 mg/g) predicted post-operative AKI after adjusting for clinical variables (unadjusted RR, 8; 1.48–15.13; adjusted RR 11.71; 1.85–16.59) (Table 4). In the group aged < 2 years, the highest first post-operative ACR tertile (> 141.3 mg/g) predicted post-operative AKI in unadjusted analysis (RR 4.52; 1.71–7.67) but not after adjusting for clinical variables (RR 2.78; 0.70–6.65) (Table 4). However, in the group aged < 2 years, the first post-operative ACR tertiles were not significantly associated with the development of stage ≥ 2 AKI. Meanwhile, in the group aged ≥ 2 years, it was not possible to calculate the risk for post-operative ACR to predict stage ≥ 2 AKI due to low event numbers (Table 4).
Association of pre- and post-operative ACR with duration of stay and mortality
In the group aged < 2 years, the median duration of ICU stay and duration of hospital stay increased significantly with advancing pre-operative (p < 0.05), post-operative day 0 (p < 0.05), post-operative day 1 (p < 0.001) and post-operative day 2 (p < 0.05) ACR levels (Table 5). Similarly, in the group aged ≥ 2 years, the median duration of ICU stay and duration of hospital stay increased significantly with advancing post-operative day 0 (p < 0.05), post-operative day 1 (p < 0.05) and post-operative day 2 (p < 0.001) ACR levels, but were not significantly associated with pre-operative ACR levels (Table 5). There were two deaths in the group aged < 2 years, the median (IQR) ACR level at post-operative day 2 was significantly higher among those who died as compared to that among those who did not die (419 (298–540) vs. 82 (58–180) mg/g; p < 0.05). Also, median (IQR) ACR levels at different time points were higher among those who died vs. did not die; however, there was a lack of statistical significance, probably due to limited sample size in the mortality group. There was one death in the age group ≥ 2 years: the highest ACR was observed on POD1, which was higher than those who did not die (350 vs. median IQR 61 (25–90.5) mg/g).
Table 6 displays the diagnostic characteristics of pre-operative and first post-operative ACR thresholds for AKI diagnosis. For the pre-operative ACR and first post-operative ACR, the ACR threshold value with maximal sensitivity and specificity in the group aged ≥ 2 years was almost one-third and three-fifths of the similar value in the group aged < 2 years, respectively. In general, negative predictive values were high while positive predictive values were low for different ACR threshold values (Table 6).
Added benefit of pre- and first post-operative ACR to clinical predictors to predict AKI
For the group aged < 2 years, AUC (95% CI) of pre-operative clinical model (0.761 (0.657–0.866)) for AKI prediction was relatively higher than pre-operative ACR alone (0.729 (0.618–0.840)), first post-operative ACR alone (0.704 (0.586–0.822)) and pre-operative ACR + first post-operative ACR (0.751 (0.645–0.856)). Similarly, for the group aged ≥ 2 years, AUC (95% CI) of pre-operative clinical model (0.842 (0.716–0.969)) was relatively higher than pre-operative ACR alone (0.639 (0.456–0.821)), first post-operative ACR alone (0.803 (0.618–0.987)) and pre-operative ACR + first post-operative ACR (0.688 (0.506–0.869)). A relatively higher increase in area under the ROC curve was observed by adding pre-operative ACR (< 2 years: 0.805 (0.713–0.896); ≥ 2 years: 0.872 (0.772–0.973)) than post-operative ACR (< 2 years: 0.777 (0.676–0.879); ≥ 2 years: 0.844 (0.718–0.969)) to the clinical model. Also, it was observed that the area under the ROC curve for clinical model + pre-operative ACR (< 2 years: 0.805 (0.713–0.896); ≥ 2 years: 0.872 (0.772–0.973)) was slightly higher than that for clinical model + pre-operative ACR + first post-operative ACR (< 2 years: 0.799 (0.705–0.893); ≥ 2 years: 0.862 (0.751–0.974)), indicating no incremental value of adding post-operative ACR in predicting AKI (Table 7).
Discussion
This study identifies that prediction of developing AKI in children post cardiac surgery using cardiopulmonary bypass increases with use of the pre-operative. The clinical model had the highest AUC in comparison to pre-operative and/or first post-operative ACR alone in predicting AKI. A combination of clinical model and - pre-operative ACR produced the best performance in AKI prediction, providing for a collective assessment of AKI risk in children undergoing open cardiac surgery. An early evidence of AKI can help in change of practices which may exacerbate kidney dysfunction, such as use of nephrotoxic drugs like aminoglycosides or NSAIDs, judicious use of contrast, optimisation of volume status and allow for prognostication and timely interventions.
Scores based on clinical variables have been previously used and validated for assessing AKI risk in settings of cardiac surgery [20, 21]. As discussed by Tinica and colleagues in a systematic review of AKI determinants after cardiac surgery in adults, most clinical scores are often able to identify severe forms of AKI but may miss mild cases and incorporation of biomarkers such as proteinuria among others would help in improving AKI risk ascertainment [22]. Zappitelli et al. in a similar study assessing albuminuria in a paediatric population following open cardiac surgery also identified a better AKI prediction model when utilising clinical variables, first post-operative ACR and cystatin C [14]. Koyner et al. were also able to demonstrate a greater determination of AKI severity progression post cardiac surgery in adults with use of biomarkers such as plasma NGAL, urine ACR and urine IL-18 over risk scores. Plasma NGAL performed best followed by urine ACR (odds ratio of 3.4) [23].
The median albuminuria in age group < 2 years with AKI was also significantly higher at all time points (pre- and post-operatively) in comparison to those without AKI, as opposed to children ≥ 2 years with AKI only showing a significant difference in the first early post-operative ACR. However, the pre-operative ACR was unable to predict AKI in adjusted analyses for either age group. Incorporation of clinical model to preoperative ACR had the highest AUC for prediction of AKI. Similar findings have been seen in adults wherein pre-operative proteinuria has shown significant association with subsequent AKI following both cardiac and non-cardiac surgeries [24,25,26], with risk increasing in tandem with greater levels of proteinuria. As comorbidities such as diabetes mellitus, hypertension and their complications tend to accrue with age, higher pre-operative proteinuria in adults with subclinical kidney impairment is plausible. In children, it is known that congenital heart diseases may accompany kidney abnormalities. Moreover, it is known that presence of congenital heart disease leads to altered physiology states, hemodynamic and hypoxic insults which can be the cause of pre-existing renal impairment in this vulnerable population [24].
Post-operatively, ACR rose in both age groups irrespective of development of AKI, possibly as a result of inflammatory or hemodynamic stress. In children > 2 years, the highest tertile (> 75.8 mg/g) of the first post-operative ACR was also able to predict AKI even after adjusting for clinical parameters (adjusted RR 11.71; CI 1.85–16.59); in children under 2 years (> 141.3 mg/g), it was able to do so in unadjusted analyses but not after adjusting for clinical factors.
In contrast, in the study conducted by Zappitelli and colleagues, the highest tertile of first post-operative ACR was able to predict stage 1 and 2 AKI even after controlling for clinical variables in all age groups [14]. Devarajan et al. also performed urinary proteomic analysis in children post cardiac surgery to look for putative biomarkers. They found three urinary protein peaks, corresponding to α1-microglobulin, α1-acid glycoprotein and albumin, to be significantly increased in children who developed AKI (increase 2 h post-surgery from baseline and checked at various time points up to 72 h); these findings were confirmed in a validation set [27].
Increased ACR levels also were significantly associated with longer ICU and hospital stays in all age groups, which signifies that kidney injury even when subtle can increase morbidity and affect outcomes adversely. Due to low event numbers (3), the data on mortality was not definitive. Devarajan and colleagues also found increased duration of hospital stay and AKI with increasing quartiles of three urinary biomarkers (including albumin) post open cardiac surgery in children [27].
In comparison with the Zappitelli et al. study, our study highlights the importance of adding pre-operative rather than post-operative ACR to the clinical model in predicting AKI, which is also reported in adults [13]. Our study also demonstrates that there is no incremental value of adding post-operative ACR for AKI prediction, while Zappitelli et al. found no improvement by incorporating pre-operative ACR in first post-operative ACR performance in any of the models. Additionally, the AUCs reported in our study are relatively higher but within the 95% CI interval reported by Zappitelli et al. For the clinical predictive model, some additional variables were considered in our study, such as previous cardiac surgeries, type of heart surgery and pre-operative creatinine as compared to that by Zappitelli et al. However, other serum or urinary biomarkers were not considered for the analysis as was done by Zappitelli et al. [13, 15].
The strengths of the present study are its prospective nature and standardised laboratory assessments of albuminuria. Our study had a few limitations including low number of events especially in older children. Being a single centre study, it affects the generalisability of its results, especially in non-cardiac settings. We did not use other serum or urinary biomarkers in our analysis which could have provided greater information.
Conclusion
This study has enhanced the use of albuminuria in AKI prediction. Preoperative ACR when added to a clinical model helps predict AKI in all age groups. It allows for AKI risk evaluation, allowing for initiation of preventive measures, better monitoring and prognostication and thus better management of children undergoing open cardiac surgery. It provides evidence for use of albuminuria as a feasible biomarker in AKI and studies assessing the same in different risk populations could provide further corroborating evidence.
Abbreviations
- AKI:
-
Acute kidney injury
- RAI:
-
Renal angina index
- ACR:
-
Urinary albumin:creatinine ratio
- KDIGO:
-
Kidney Disease Improving Global Outcomes
- CPB:
-
Cardiopulmonary bypass
- RACHS-1:
-
Risk adjustment for congenital heart surgery
- AUC:
-
Area under the curve
- POD:
-
Post-operative day
- NGAL:
-
Neutrophil gelatinase–associated lipocalin
References
Li S, Krawczeski CD, Zappitelli M, Devarajan P, Thiessen-Philbrook H, Coca SG, Kim RW, Parikh CR (2011) Incidence, risk factors, and outcomes of acute kidney injury after pediatric cardiac surgery – a prospective multicenter study. Crit Care Med 39:1493–1499
Mao H, Katz N, Ariyanon W, Blanca-Martos L, Adýbelli Z, Giuliani A (2013) Cardiac surgery associated acute kidney injury. Cardiorenal Med 3:178–199
Zappitelli M, Bernier P-L, Saczkowski RS, Tchervenkov CI, Gottesman R, Dancea A (2009) A small post-operative rise in serum creatinine predicts acute kidney injury in children undergoing cardiac surgery. Kidney Int 76:885–892
Alam S, Shalini A, Hegde R, Mazahir R (2017) Acute kidney injury as a predictor of poor outcome post cardiopulmonary bypass in children. Int Contemp Pediatr 4:234–240
Ostermann M, Zarbock A, Goldstein S, Kashani K, Macedo E, Murugan R, Bell M, Forni L, Guzzi L, Joannidis M, Kane-Gill SL, Legrand M, Mehta R, Murray PT, Pickkers P, Plebani M, Prowle J, Ricci Z, Rimmelé T, Rosner M, Shaw AD, Kellum JA, Ronco C (2020) Recommendations on acute kidney injury biomarkers from the Acute Disease Quality Initiative Consensus Conference: a consensus statement. JAMA Netw Open 3:e2019209. https://doi.org/10.1001/jamanetworkopen.2020.19209
Menon S, Goldstein SL, Mottes T, Fei L, Kaddourah A, Terrell T, Arnold P, Bennett MR, Basu RK (2016) Urinary biomarker incorporation into the renal angina index early in intensive care unit admission optimizes acute kidney injury prediction in critically ill children: a prospective cohort study. Nephrol Dial Transplant 31:586–594
Meersch M, Schmidt C, Hoffmeier A, Van Aken H, Wempe C, Gerss J, Zarbock A (2017) Prevention of cardiac surgery-associated AKI by implementing the KDIGO guidelines in high risk patients identified by biomarkers: the PrevAKI randomized controlled trial. Intensive Care Med 43:1551–1561
Levey AS, de Jong PE, Coresh J, El Nahas M, Astor BC, Matsushita K, Gansevoort RT, Kasiske BL, Eckardt KU (2011) The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney Int 80:17–28
Ware LB, Johnson AC, Zager RA (2011) Renal cortical albumin gene induction and urinary albumin excretion in response to acute kidney injury. Am J Physiol Renal Physiol 300:F628-638
Parr SK, Matheny ME, Abdel-Kader K, Greevy RA Jr, Bian A, Fly J, Chen G, Speroff T, Hung AM, Ikizler TA, Siew ED (2018) Acute kidney injury is a risk factor for subsequent proteinuria. Kidney Int 93:460–469
Yu Y, Jin H, Holder D, Ozer JS, Villarreal S, Shughrue P, Shi S, Figueroa DJ, Clouse H, Su M, Muniappa N, Troth SP, Bailey W, Seng J, Aslamkhan AG, Thudium D, Sistare FD, Gerhold DL (2010) Urinary biomarkers trefoil factor 3 and albumin enable early detection of kidney tubular injury. Nat Biotechnol 28:470–477
Coca SG, Jammalamadaka D, Sint K, Thiessen Philbrook H, Shlipak MG, Zappitelli M, Devarajan P, Hashim S, Garg AX, Parikh CR, Translational Research Investigating Biomarker Endpoints in Acute Kidney Injury Consortium (2012) Preoperative proteinuria predicts acute kidney injury in patients undergoing cardiac surgery. J Thorac Cardiovasc Surg 143:495–502
Huang TM, Wu VC, Young GH, Lin YF, Shiao CC, Wu PC, Li WY, Yu HY, Hu FC, Lin JW, Chen YS, Lin YH, Wang SS, Hsu RB, Chang FC, Chou NK, Chu TS, Yeh YC, Tsai PR, Huang JW, Lin SL, Chen YM, Ko WJ, Wu KD (2011) National Taiwan University Hospital Study Group of Acute Renal Failure. Preoperative proteinuria predicts adverse renal outcomes after coronary artery bypass grafting. J Am Soc Nephrol 22:156–163
Zappitelli M, Coca SG, Garg AX, Krawczeski CD, Thiessen-Philbrook HT, Sint K, Li S, Parikh CR, Devarajan P (2012) The association of albumin/creatinine ratio with postoperative AKI in children undergoing cardiac surgery. Clin J Am Soc Nephrol 7:1761–1769
Kidney Diseases Improving Global Outcomes (2012) KDIGO clinical practice guideline for acute kidney injury. Kidney Int 2(Suppl 1):1–138
Schwartz GJ, Muñoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL (2009) New equations to estimate GFR in children with CKD. J Am Soc Nephrol 20:629–637
Jenkins KJ, Gauvreau K, Newburger JW, Spray TL, Moller JH, Iezzoni LI (2002) Consensus-based method for risk adjustment for surgery for congenital heart disease. J Thorac Cardiovasc Surg 123:110–118
Goldstein SL, Currier H, Graf JM, Cosio CC, Brewer ED, Sachdeva R (2007) Outcome in children receiving continuous venovenous hemofiltration. Pediatrics 107:1309–1312
Hoffman TM, Wernovsky G, Atz AM, Bailey JM, Akbary A, Kocsis JF, Nelson DP, Chang AC, Kulik TJ, Spray TI, Wessel DL (2002) Prophylactic intravenous use of milrinone after cardiac operation in pediatrics (PRIMACORP) study. Prophylactic Intravenous Use of Milrinone After Cardiac Operation in Pediatrics. Am Heart J 143:15–21
Thakar CV, Arrigain S, Worley S, Yared J-P, Paganini EP (2005) A clinical score to predict acute renal failure after cardiac surgery. J Am Soc Nephrol 16:162–168
Mehta RH, Grab JD, O’Brien SM, Bridges CR, Gammie JS, Haan CK, Ferguson TB, Peterson ED; Society of Thoracic Surgeons National Cardiac Surgery Database Investigators (2006) Bedside tool for predicting the risk of postoperative dialysis in patients undergoing cardiac surgery. Circulation 114:2208–2216
Tinica G, Brinza C, Covic A, Popa IV, Tarus A, Bacusca AE, Burlacu A (2020) Determinants of acute kidney injury after cardiac surgery: a systematic review. Rev Cardiovasc Med 21:601–610
Koyner JL, Garg AX, Coca SG, Sint K, Thiessen-Philbrook H, Patel UD, Shlipak MG, Parikh CR; TRIBE-AKI Consortium (2012) Biomarkers predict progression of acute kidney injury after cardiac surgery. J Am Soc Nephrol 23:905–914
Zheng J, Yao Y, Han L, Xiao Y (2013) Renal function and injury in infants and young children with congenital heart disease. Pediatr Nephrol 28:99–104
Wahl TS, Graham LA, Morris MS, Richman JS, Hollis RH, Jones CE, Itani KM, Wagner TD, Mull HJ, Whittle JC, Telford GL, Rosen AK, Copeland LA, Burns EA, Hawn MT (2018) Association between preoperative proteinuria and postoperative acute kidney injury and readmission. JAMA Surg 153:e182009
Park S, Lee S, Lee A, Paek JH, Chin HJ, Na KY, Chae DW, Kim S (2018) Preoperative dipstick albuminuria and other urine abnormalities predict acute kidney injury and patient outcomes. Surgery 163:1178–1185
Devarajan P, Krawczeski CD, Nguyen MT, Kathman T, Wang Z, Parikh CR (2010) Proteomic identification of early biomarkers of acute kidney injury after cardiac surgery in children. Am J Kidney Dis 56:632–642
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Both Dr. Arushi Nautiyal and Dr. Sidharth Kumar Sethi contributed equally and shall be first authors.
Supplementary Information
Below is the link to the electronic supplementary material.

Rights and permissions
About this article
Cite this article
Nautiyal, A., Sethi, S.K., Sharma, R. et al. Perioperative albuminuria and clinical model to predict acute kidney injury in paediatric cardiac surgery. Pediatr Nephrol 37, 881–890 (2022). https://doi.org/10.1007/s00467-021-05219-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-021-05219-0