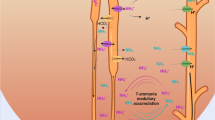
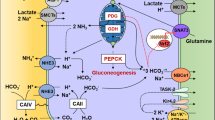
Abstract
The importance of renal ammonia metabolism in acid–base homeostasis is well known. However, the effects of renal ammonia metabolism other than in acid–base homeostasis are not as widely recognized. First, ammonia differs from almost all other solutes in the urine in that it does not result from arterial delivery. Instead, ammonia is produced by the kidney, and only a portion of the ammonia produced is excreted in the urine, with the remainder returned to the systemic circulation through the renal veins. In normal individuals, systemic ammonia addition is metabolized efficiently by the liver, but in patients with either acute or chronic liver disease, conditions that increase the addition of ammonia of renal origin to the systemic circulation can result in precipitation and/or worsening of hyperammonemia. Second, ammonia appears to serve as an intrarenal paracrine signaling molecule. Hypokalemia increases proximal tubule ammonia production and secretion as well as reabsorption in the thick ascending limb of the loop of Henle, thereby increasing delivery to the renal interstitium and the collecting duct. In the collecting duct, ammonia decreases potassium secretion and stimulates potassium reabsorption, thereby decreasing urinary potassium excretion and enabling feedback correction of the initiating hypokalemia. Finally, the stimulation of renal ammonia metabolism by hypokalemia may contribute to the development of metabolic alkalosis, which in turn can stimulate NaCl reabsorption and contribute to the intravascular volume expansion, increased blood pressure and diuretic resistance that can develop with hypokalemia. The evidence supporting these novel non-acid–base roles of renal ammonia metabolism is discussed in this review.








Similar content being viewed by others
Notes
Ammonia can exist in two molecular forms, NH3 (free ammonia) and NH4 + (ammonium cation). Throughout this review, “ammonia” refers to the combination of both molecules; “NH3” refers specifically to the molecular form of NH3; “NH4 +” refers specifically to the molecular form NH4 +.
References
Gil-Pena H, Mejia N, Santos F (2013) Renal tubular acidosis. J Pediatr 164:691–698
Mitch WE (2006) Metabolic and clinical consequences of metabolic acidosis. J Nephrol 19:S70–S75
Weiner ID, Verlander JW (2015) Renal acidification mechanisms. In: Tall MW, Chertow GM, Marsden PA, Skorecki K, Yu AS, Brenner BM (eds) Brenner and Rector’s the kidney, 10th edn. W.B. Saunders Press, New York, pp 234–257
Hamm LL, Nakhoul N, Hering-Smith KS (2015) Acid–base homeostasis. Clin J Am Soc Nephrol 10:2232–2242
Weiner ID, Mitch WE, Sands JM (2014) Urea and ammonia metabolism and the control of renal nitrogen excretion. Clin J Am Soc Nephrol 10:1444–1458
Weiner ID, Verlander JW (2013) Renal ammonia metabolism and transport. Compr Physiol 3:201–220
Halperin ML, Dhadli SC, Kamel KS (2006) Physiology of acid–base balance: links with kidney stone prevention. Semin Nephrol 26:441–446
Unwin RJ, Capasso G, Shirley DG (2004) An overview of divalent cation and citrate handling by the kidney. Nephron Physiol 98:15–20
Eriksson LS, Broberg S, Bjorkman O, Wahren J (1985) Ammonia metabolism during exercise in man. Clin Physiol 5:325–336
Elkinton JR, Huth EJ, Webster GD Jr, McCance RA (1960) The renal excretion of hydrogen ion in renal tubular acidosis. Am J Med 36:554–575
Curthoys NP, Moe OW (2014) Proximal tubule function and response to acidosis. Clin J Am Soc Nephrol 9:1627–1638
Wright PA, Knepper MA (1990) Phosphate-dependent glutaminase activity in rat renal cortical and medullary tubule segments. Am J Physiol 259:F961–F970
Wright PA, Knepper MA (1990) Glutamate dehydrogenase activities in microdissected rat nephron segments: effects of acid–base loading. Am J Physiol 259:F53–F59
Nagami GT (2004) Ammonia production and secretion by S3 proximal tubule segments from acidotic mice: role of ANG II. Am J Physiol Renal Physiol 287:F707–F712
Nagami GT, Sonu CM, Kurokawa K (1986) Ammonia production by isolated mouse proximal tubules perfused in vitro: effect of metabolic acidosis. J Clin Invest 78:124–129
Nagami GT (2008) Role of angiotensin II in the enhancement of ammonia production and secretion by the proximal tubule in metabolic acidosis. Am J Physiol Renal Physiol 294:F874–F880
Weiner ID, Verlander JW (2014) Ammonia transport in the kidney by Rhesus glycoproteins. Am J Physiol Renal Physiol 306:F1107–F1120
Weiner ID, Verlander JW (2011) Role of NH3 and NH4+ transporters in renal acid–base transport. Am J Physiol Renal Physiol 300:F11–F23
Owen EE, Tyor MP, Flanagan JF, Berry JN (1960) The kidney as a source of blood ammonia in patients with liver disease: the effect of acetazolamide. J Clin Invest 39:288–294
Haussinger D, Lamers WH, Moorman AF (1992) Hepatocyte heterogeneity in the metabolism of amino acids and ammonia. Enzyme 46:72–93
Haussinger D (1989) Glutamine metabolism in the liver: overview and current concepts. Metabolism 38:14–17
Kaiser S, Gerok W, Haussinger D (1988) Ammonia and glutamine metabolism in human liver slices: new aspects on the pathogenesis of hyperammonaemia in chronic liver disease. Eur J Clin Invest 18:535–542
Haussinger D (1987) Structural-functional organization of hepatic glutamine and ammonium metabolism. Biochem Soc Trans 15:369–372
Haussinger D (1986) Regulation of hepatic ammonia metabolism: the intercellular glutamine cycle. Adv Enzyme Regul 25:159–180
Verlander JW, Chu D, Lee HW, Handlogten ME, Weiner ID (2013) Expression of glutamine synthetase in the mouse kidney: localization in multiple epithelial cell types and differential regulation by hypokalemia. Am J Physiol Renal Physiol 305:F701–F713
Koen H, Okuda K, Musha H, Tateno Y, Fukuda N, Matsumoto T, Shisido F, Rikitake T, Iinuma T, Kurisu A, Arimizu N (1980) A dynamic study of rectally absorbed ammonia in liver cirrhosis using ammonia and a positron camera. Dig Dis Sci 25:842–848
Cooper AJ (1990) Ammonia metabolism in normal and portacaval-shunted rats. Adv Exp Med Biol 272:23–46
Conn HO (1972) Studies of the source and significance of blood ammonia. IV. Early ammonia peaks after ingestion of ammonium salts. Yale J Biol Med 45:543–549
Gumz ML, Rabinowitz L, Wingo CS (2015) An Integrated view of potassium homeostasis. N Engl J Med 373:60–72
Unwin RJ, Luft FC, Shirley DG (2011) Pathophysiology and management of hypokalemia: a clinical perspective. Nat Rev Nephrol 7:75–84
Weiner ID, Wingo CS (1997) Hypokalemia—consequences, causes and correction. J Am Soc Nephrol 8:1179–1188
Gabuzda GJ, Hall II (1966) Relation of potassium depletion to renal ammonium metabolism and hepatic coma. Medicine (Baltimore) 45:481–489
Shear L, Gabuzda GJ (1970) Potassium deficiency and endogenous ammonium overload from kidney. Am J Clin Nutr 23:614–618
Baertl JM, Sancetta SM, Gabuzda GJ (1963) Relation of acute potassium depletion to renal ammonium metabolism in patients with cirrhosis. J Clin Invest 42:696–706
Han KH, Lee HW, Handlogten ME, Bishop JM, Levi M, Kim J, Verlander JW, Weiner ID (2011) Effect of hypokalemia on renal expression of the ammonia transporter family members, Rh B glycoprotein and Rh C glycoprotein, in the rat kidney. Am J Physiol Renal Physiol 301:F823–F832
Busque SM, Wagner CA (2009) Potassium restriction, high protein intake, and metabolic acidosis increase expression of the glutamine transporter SNAT3 (Slc38a3) in mouse kidney. Am J Physiol Renal Physiol 297:F440–F450
Hossain SA, Chaudhry FA, Zahedi K, Siddiqui F, Amlal H (2011) Cellular and molecular basis of increased ammoniagenesis in potassium deprivation. Am J Physiol Renal Physiol 301:F969–F978
Nagami GT (1990) Effect of bath and luminal potassium concentration on ammonia production and secretion by mouse proximal tubules perfused in vitro. J Clin Invest 86:32–39
Adam WR, Koretsky AP, Weiner MW (1986) 31P-NMR in vivo measurement of renal intracellular pH: effects of acidosis and K+ depletion in rats. Am J Physiol 251:F904–F910
Jones B, Simpson DP (1983) Influence of alterations in acid–base conditions on intracellular pH of intact renal cortex. Ren Physiol 6:28–35
Schoolwerth AC, Culpepper RM (1990) Measurement of intracellular pH in suspensions of renal tubules from potassium-depleted rats. Miner Electrolyte Metab 16:191–196
Olde Damink SW, Jalan R, Deutz NE, Redhead DN, Dejong CH, Hynd P, Jalan RA, Hayes PC, Soeters PB (2003) The kidney plays a major role in the hyperammonemia seen after simulated or actual GI bleeding in patients with cirrhosis. Hepatology 37:1277–1285
Bounoure L, Ruffoni D, Muller R, Kuhn GA, Bourgeois S, Devuyst O, Wagner CA (2014) The role of the renal ammonia transporter Rhcg in metabolic responses to dietary protein. J Am Soc Nephrol 25:2040–2052
Brosnan JT, McPhee P, Hall B, Parry DM (1978) Renal glutamine metabolism in rats fed high-protein diets. Am J Physiol 235:E261–E265
Lee HW, Osis G, Handlogten ME, Guo H, Verlander JW, Weiner ID (2015) Effect of dietary protein restriction on renal ammonia metabolism. Am J Physiol Renal Physiol 308:F1463–F1473
Inoue K, Takahashi T, Yamamoto Y, Suzuki E, Takahashi Y, Imai K, Inoue Y, Hirai K, Tsuji D, Itoh K (2015) Influence of glutamine synthetase gene polymorphisms on the development of hyperammonemia during valproic acid–based therapy. Seizure 33:76–80
Elhamri M, Ferrier B, Martin M, Baverel G (1993) Effect of valproate, sodium 2-propyl-4-pentenoate and sodium 2-propyl-2-pentenoate on renal substrate uptake and ammoniagenesis in the rat. J Pharmacol Exp Ther 266:89–96
Martin G, Durozard D, Besson J, Baverel G (1990) Effect of the antiepileptic drug sodium valproate on glutamine and glutamate metabolism in isolated human kidney tubules. Biochim Biophys Acta 1033:261–266
Doval M, Culebras M, Lopez-Farre A, Rengel M, Gougoux A, Vinay P, Lopez-Novoa JM (1989) Effect of valproate on lactate and glutamine metabolism by rat renal cortical tubules. Proc Soc Exp Biol Med 190:357–364
Marini AM, Zaret BS, Beckner RR (1988) Hepatic and renal contributions to valproic acid-induced hyperammonemia. Neurology 38:365–371
Rengel M, Doval M, Culebras M, Gougoux A, Vinay P, Lopez-Novoa JM (1988) Ammoniagenesis and valproic acid in the rat in vivo: role of the kidney. Contrib Nephrol 63:132–135
Imler M, Chabrier G, Marescaux C, Warter JM (1986) Effects of 2,4-dinitrophenol on renal ammoniagenesis in the rat. Eur J Pharmacol 123:175–179
Warter JM, Brandt C, Marescaux C, Rumbach L, Micheletti G, Chabrier G, Krieger J, Imler M (1983) The renal origin of sodium valproate-induced hyperammonemia in fasting humans. Neurology 33:1136–1140
Warter JM, Marescaux C, Brandt C, Rumbach L, Micheletti G, Chabrier G, Imler M, Kurtz D (1983) Sodium valproate associated with phenobarbital: effects on ammonia metabolism in humans. Epilepsia 24:628–633
Warter JM, Imler M, Marescaux C, Chabrier G, Rumbach L, Micheletti G, Krieger J (1983) Sodium valproate-induced hyperammonemia in the rat: Role of the kidney. Eur J Pharmacol 87:177–182
Tannen RL (1977) Relationship of renal ammonia production and potassium homeostasis. Kidney Int 11:453–465
Tannen RL (1970) The effect of uncomplicated potassium depletion on urine acidification. J Clin Invest 49:813–827
Tannen RL, Terrien T (1975) Potassium-sparing effect of enhanced renal ammonia production. Am J Physiol 228:699–705
Jaeger P, Karlmark B, Giebisch G (1983) Ammonium transport in rat cortical tubule: relationship to potassium metabolism. Am J Physiol 245:F593–F600
Hamm LL, Gillespie C, Klahr S (1985) NH4Cl inhibition of transport in the rabbit cortical collecting tubule. Am J Physiol 248:F631–F637
Wang WH, Giebisch G (2009) Regulation of potassium (K) handling in the renal collecting duct. Pflugers Arch 458:157–168
Muto S (2001) Potassium transport in the mammalian collecting duct. Physiol Rev 81:85–116
Frank AE, Wingo CS, Weiner ID (2000) Effects of ammonia on bicarbonate transport in the cortical collecting duct. Am J Physiol Renal Physiol 278:F219–F226
Chalfant ML, Denton JS, Berdiev BK, Ismailov II, Benos DJ, Stanton BA (1999) Intracellular H+ regulates the alpha-subunit of ENaC, the epithelial Na+ channel. Am J Physiol 276:C477–C486
Konstas AA, Mavrelos D, Korbmacher C (2000) Conservation of pH sensitivity in the epithelial sodium channel (ENaC) with Liddle’s syndrome mutation. Pflugers Arch 441:341–350
Nakhoul NL, Hering-Smith KS, Abdulnour-Nakhoul SM, Hamm LL (2001) Ammonium interaction with the epithelial sodium channel. Am J Physiol Renal Physiol 281:F493–F502
Greenlee MM, Lynch IJ, Gumz ML, Cain BD, Wingo CS (2010) The renal H, K-ATPases. Curr Opin Nephrol Hypertens 19:478–482
Weiner ID, Linus S, Wingo CS (2010) Disorders of potassium metabolism. In: Johnson RJ, Fluege J, Feehally J (eds) Comprehensive clinical nephrology, 4th edn. W.B. Saunders, Philadelphia, pp 118–129
Frank AE, Wingo CS, Andrews PM, Ageloff S, Knepper MA, Weiner ID (2002) Mechanisms through which ammonia regulates cortical collecting duct net proton secretion. Am J Physiol Renal Physiol 282:F1120–F1128
Good DW (1990) Ammonium transport by the loop of Henle. Miner Electrolyte Metab 16:291–298
Wall SM, Davis BS, Hassell KA, Mehta P, Park SJ (1999) In rat tIMCD, NH4 + uptake by the Na+, K+-ATPase is critical to net acid secretion during chronic hypokalemia. Am J Physiol 277:F866–F874
Frank AE, Weiner ID (2001) Effects of ammonia on acid–base transport by the B-type intercalated cell. J Am Soc Nephrol 12:1607–1614
Barri YM, Wingo CS (1997) The effects of potassium depletion and supplementation on blood pressure: a clinical review. Am J Med Sci 314:37–40
Krishna GG, Kapoor SC (1991) Potassium depletion exacerbates essential hypertension. Ann Intern Med 115:77–83
Krishna GG (1990) Effect of potassium intake on blood pressure. J Am Soc Nephrol 1:43–52
Hernandez RE, Schambelan M, Cogan MG, Colman J, Morris RC, Sebastian A (1987) Dietary NaCl determines severity of potassium depletion-induced metabolic alkalosis. Kidney Int 31:1356–1367
Cremer W, Bock KD (1977) Symptoms and course of chronic hypokalemic nephropathy in man. Clin Nephrol 7:112–119
Loon NR, Wilcox CS (1998) Mild metabolic alkalosis impairs the natriuretic response to bumetanide in normal human subjects. Clin Sci (Lond) 94:287–292
Tokonami N, Morla L, Centeno G, Mordasini D, Ramakrishnan SK, Nikolaeva S, Wagner CA, Bonny O, Houillier P, Doucet A, Firsov D (2013) a-Ketoglutarate regulates acid–base balance through an intrarenal paracrine mechanism. J Clin Invest 123:3166–3171
Martin M, Ferrier B, Baverel G (1989) Transport and utilization of alpha-ketoglutarate by the rat kidney in vivo. Pflugers Arch 413:217–224
Ferrier B, Martin M, Baverel G (1985) Reabsorption and secretion of alpha-ketoglutarate along the rat nephron: a micropuncture study. Am J Physiol 248:F404–F412
Balagura S, Pitts RF (1964) Renal handling of a-ketoglutarate by the dog. Am J Physiol 207:483–494
Terker AS, Zhang C, Erspamer KJ, Gamba G, Yang CL, Ellison DH (2016) Unique chloride-sensing properties of WNK4 permit the distal nephron to modulate potassium homeostasis. Kidney Int 89:127–134
Hadchouel J, Ellison DH, Gamba G (2016) Regulation of renal electrolyte transport by WNK and SPAK-OSR1 kinases. Annu Rev Physiol 78:367–389
Zhang C, Wang L, Zhang J, Su XT, Lin DH, Scholl UI, Giebisch G, Lifton RP, Wang WH (2014) KCNJ10 determines the expression of the apical Na-Cl cotransporter (NCC) in the early distal convoluted tubule (DCT1). Proc Natl Acad Sci USA 111:11864–11869
Subramanya AR, Ellison DH (2014) Distal convoluted tubule. Clin J Am Soc Nephrol 9:2147–2163
Bazua-Valenti S, Chavez-Canales M, Rojas-Vega L, Gonzalez-Rodriguez X, Vazquez N, Rodriguez-Gama A, Argaiz ER, Melo Z, Plata C, Ellison DH, Garcia-Valdes J, Hadchouel J, Gamba G (2015) The effect of WNK4 on the Na+−Cl- cotransporter is modulated by intracellular chloride. J Am Soc Nephrol 26:1781–1786
Acknowledgments
Generation and publication of this review was supported by funds from the NIH (R01–DK045788 and R01–DK107798).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that he has no conflict of interest.
Rights and permissions
About this article
Cite this article
Weiner, I.D. Roles of renal ammonia metabolism other than in acid–base homeostasis. Pediatr Nephrol 32, 933–942 (2017). https://doi.org/10.1007/s00467-016-3401-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-016-3401-x