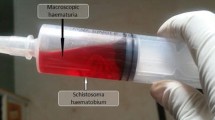
Abstract
Schistosomiasis is the second most common socio-economically devastating parasitic disease after malaria, affecting about 240 million residents of developing countries. In Africa, it predominantly manifests as urogenital disease, and the main infective agent is Schistosoma hematobium. Endemicity is propagated by poor socio-economic status and environmental degradation due to rapid urbanization. Recreational swimming is a potent medium for the spread of disease in children and adolescents. Most affected individuals are asymptomatic. The male and female worms are equipped with an extraordinary capacity for immune evasion and are able to co-habit for several decades within the pelvic venous plexus. Eggs deposited in the bladder wall resist elimination by type 1 T lymphocytes. Instead, they are sustained by pro-fibrogenic encapsulation (as modulated by type 2 helper cells). Progressive bladder disease results in obstructive uropathy and predisposes to (mostly) squamous cell carcinoma. Schistosomal glomerulopathy manifests as a clinical spectrum of asymptomatic proteinuria, nephrosis and/or nephritic syndrome. Findings on renal biopsy may be influenced by co-morbidity with Salmonella bacteria, amyloidosis and hepatitis C infection. Potentially fatal Katayama fever and spinal radiculopathy may ensue in tourists visiting an endemic zone. Early detection by urine microscopy is hampered by low urinary excretion rates of the parasite eggs. Although useful in travelers with newly acquired disease, the results of the serological antibody assay may be false positive in residents of an endemic zone. Cystoscopy, however, may be invaluable. Due to its safety, effectiveness and once-daily dosing, praziquantel is the drug of choice. An integrated approach that includes mass chemotherapy, environmental health programs and public health education is the most cost-effective preventive strategy.
Similar content being viewed by others
Urinary schistosomiasis
Epidemiology
After malaria, schistosomiasis is the second most common socio-economically devastating tropical parasitic disease. Parasite infestation has been documented in 78 countries of Africa, Asia, the Middle East and South America [1]. Despite the availability of effective drugs, the annual death rate is around 200,000 in sub-Sahara Africa alone, making the group of parasites which cause schistosomiasis the most lethal worms in the world. The majority of human disease is mediated by Schistosoma hematobium, S. mansoni and S. japonicum [2]. Each of these species has a tropism for different body organs, with S. hematobium being the main cause of urogenital disease [2, 3]. Poor access to economic opportunity accounts for the uneven distribution of infection in endemic regions. Subsistence farming, inadequate water supply, poor public sanitation, rapid urbanization and dam construction are common predisposing factors [3]. Although parasite infection has been reported in early infancy, peak incidence occurs in early adolescence as a result of frequent bathing in contaminated pools of water [4]. Apart from lower exposure in adults, the capacity to resist new infection by eosinophil secretion of antigen-specific immunoglobulin E (IgE) is age dependent [5]. Children younger than 13 years have a higher serum level of IgM, IgG2 and IgG4 isotypes which block the protective effect of IgE [6].
Biological life cycle
Schistosoma hematobium is a digenean trematode that uses humans as the definitive host and molluscs (genus Bulinus) as the intermediate host (Fig. 1). Its life cycle begins with urinary excretion of eggs of the trematode into freshwater populated by susceptible snails. The eggs are then hatched to release motile, non-feeding, ciliated miracidiae that survive for only 7 days. After penetrating the water snail, the larvae replicate to form sporocysts which produce thousands of free swimming cercariae [2] which are able to penetrate the skin of a human within a few seconds of contact. Once in the human body, they are transformed into schistosomula which enter the circulatory system and migrate through the lungs to the liver where they mature into adult male or female worms. Bound by the male gynecophoric groove, both types of worms move to the pelvic venous plexus for 3–5 years of reproductive co-habitation [7].
Immunopathology
The migrating larva avoids destruction by the membrane attack complex (C5–C9) by rapidly shedding its glycocalyx outer covering [8]. During migration, the schistosomule adopts a host-derived outer membrane to prevent elimination by type 1 helper cells. Of the eggs produced, 50 % are not excreted but trapped in the bladder wall where they activate resident inflammatory cells to secrete tumor necrosis factor (TNF-α) and eotaxin (potently attracts eosinophils) [9]. Ultimately, there is encapsulation of the egg by granuloma formulation (Fig. 2a). An attempt to eliminate the sequestered eggs may be halted by a shift from the type 1 immune response (secretion of interferon-γ) to a pro-fibrogenic Th2 cell activity which produces interleukin (IL)-4, IL-10 and IL-13 [10]. There is concurrent transformation of classically activated macrophages (induction of nitric oxide synthase) to the alternatively activated variants (expression of arginase-1, Ym-1 and Fizz1). Over time, IL-10 and regulatory T cells downregulate the progression of chronic bladder fibrosis. This modulatory effect may be suppressed in individuals with polymorphism in the promoter gene for ficolin-2 [11].
Clinical manifestations
Transient pruritic dermatitis or swimmer’s itch (Fig. 2b) may occur in response to cercarial skin penetration [12, 13]. However, newly infected patients are often asymptomatic [7]. Common symptoms are urinary frequency, urgency, dysuria and end-stream hematuria. Of these symptoms, terminal hematuria may be the most feasible and is often the basis for epidemiologic diagnosis. Iron deficiency anemia may be exacerbated by co-morbidity with other endemic tropical diseases, such as malaria and heminthiasis. A substantial amount of iron is sequestered by vitelline cells for the formation of the parasite eggshell [14]. In addition, host iron recycling is disrupted by a pro-inflammatory synthesis of hepcidin, an acute phase reactant [15]. Although structural deficits are sometimes reversible by medical treatment, obstructive uropathy may invariably result from progressive bladder fibrosis, ureteral dilatation and hydronephrosis, particularly in older patients [16]. Ulcerations of bladder mucosa, focal bladder wall calcification and renal stone formation may result. Urological surgery is seldom required for rehabilitation. Genital disease may cause sexual dysfunction, facilitates human immunodeficiency virus transmission and promotes infertility in adolescents and young adults [17].
The ease of access to wider geographical regions for tourism has increased the infection rate among travelers [12]. Such individuals lack any acquired immunity and within 6 weeks after infection they may experience a severe hypersensitivity reaction in response to the first bout of egg antigen release by adult worms. Katayama fever is characterized by hyperpyrexia, myalgia, headache, cough, emesis and diarrhea [18]. In addition, paraplegia from transverse myelitis may result from paravertebral migration of parasite eggs. Nevertheless complete neurological recovery is feasible with early medical intervention [19].
Urinary tract infection
Due to disruption of the mucosal barrier, there is a high rate of bacterial superinfection, ranging from 30 to 80 % in endemic communities [20, 21]. Isolates are mostly regular uropathogens, but Salmonella infection is not uncommon [21]. Salmonella evades the host immune response by attaching itself to the adult worm’s surface receptors [22]. Urinary carriers may serve as a source of epidemic typhoid fever. Urine isolation of Salmonella bacteria in endemic regions should arouse suspicion for a co-morbid schistosomiasis [21, 22].
Bladder carcinoma
There is a 30-fold higher risk of developing bladder cancer, mostly squamous cell carcinoma (SCC) variant in regions of Egypt with endemic schistosomiasis [23]. A fall in the prevalence rate of schistosomiasis from 1980 to 2005 due to a public health intervention was followed by a six-fold lower rate of SCC, clearly suggesting a cause and effect relationship [24].
Schistosomal glomerulopathy
Schistosomal glomerulopathy (SGN) occurs in response to infection with both S. hematobium and S. mansoni. Apparently due to the greater occurrence of subclinical disease in patients with S. hematobium, a higher prevalence of glomerular disease is often reported in those infected with S. mansoni (Tables 1, 2) [25–27]. SGN manifests as a clinical, spectrum of asymptomatic, proteinuria, nephrosis and/or nephritic syndrome. Based on experimental and clinicopathologic data, in 1992 the African Association of Nephrology (AFRAN) recognized six categories of SGN. The most common category is Class I SGN and it is characterized by mesangial proliferative histology. Infected individuals are often asymptomatic (60 %) [26]. Class II SGN is an exudative proliferative lesion that occurs in response to Salmonella bacteria superinfection. Class III SGN has a membrano-proliferative glomerular pattern, Class IV has focal segmental glomerulosclerosis, Class V is due to secondary amyloidosis and Class VI is a mixed pathology of proliferative, focal sclerosis, amyloidosis and thrombotic cryoglobulin from a co-morbid hepatitis C infection. Except for the clinical recovery observed in patients with Class I and II SGN, patients with the other categories often progress to end-stage kidney disease by adulthood. Data in the 2008 Egyptian Renal Registry showed that SGN may account for 2.8 % of the 483 adult patients with end stage kidney disease per million of population [www.esnonline.net]. Immune complex deposits (IgM, C3, and C1q) containing adult worm antigens (gut-associated proteoglycan) in the glomerulus are the early pathological features [27]. There may be late deposits of IgG and IgA. Membrano-proliferative response to a direct localization of egg granuloma in kidney tissue is a rare event [25]. Because most immune complexes are non-nephritogenic, there is no correlation between overt kidney disease and low level of serum C3 [28].
Diagnosis
Detection of parasite eggs and motile miracidium by urine microscopy is simple, inexpensive and is considered to be the gold standard for diagnosis (Fig. 3) [29]. The result may be a false negative in the first 6 weeks prior to full maturity of the worm and due to a lower rate of egg excretion. Collection of three daily filtered urine samples after a mid-day session of physical exercise, the peak period for egg output, increases the detection rate [2]. Field surveys of end-stream hematuria, urine evaluation for blood and protein and bladder ultrasonography (assesses morbidity) are useful screening tools [2, 30, 31]. In addition, the excretion of eosinophil cationic protein in the urine (as detected by enzyme-linked immunosorbent assay) may be a biomarker of inflammatory bladder injury [30]. In cases of diagnostic challenges, cystoscopy may show a typical hemorrhagic mucosa, submucosal nodules and sandy patches (micro-granuloma). Late findings of cystoscopy are a “cooked rice grain” appearance (macro-granuloma) and erythematous fibrous polyps [31]. Bladder biopsy may show granuloma-encased eggs but may fail to demonstrate the viability of the eggs (Fig. 2a). Direct detection of miracidium-containing eggs using cystoscopy-aided confocal laser scanning microscopy produces stronger evidence of disease activity [32].
Approach to a difficult diagnosis of urogenital schistosomiasis. Due to an only intermittent excretion of the parasite in the urine, diagnosis of schistosomiasis can be challenging. Early diagnosis may prevent a fatal outcome, particularly in patients with Katayama fever (white boxes). A high index of suspicion is crucial, and a multi-diagnostic approach may improve accuracy. Due to the light parasite burden, serological diagnosis based on antibody detection of antigen derivatives of ova, adult worms and cercaria is of practical importance in infested travelers to an endemic zone. The test may be falsely negative due to late seroconversion (>6 weeks). Direct antigen detection with monoclonal antibody may improve yield. New diagnostic tools are the PCR for detection of parasite DNA in urine sample and the enzyme linked immunoabsorbent assay for detecting urine eosinophilic cationic protein (suggests active bladder infection). Direct urine microscopy is the main diagnostic tool among residents of endemic zone (black boxes). Cystoscopy may be required if non-invasive tools are unhelpful. Rectal biopsy may improve isolation of S. hematobium ova. Eggs containing miracidium indicate active bladder infection. Detection of miracidium can be facilitated by the miracidium hatching assay and a more recent development, confocal laser scanning microscopy. UTI Urinary tract infection
Due to a light parasite burden, serological assays for detection of IgG, IgM or IgE antibody response to antigen derivatives of parasite are often required for the diagnosis of infected travelers to endemic zones [33]. It is less useful diagnostic tool in an endemic community as it fails to discriminate current from previous infection, and the parasite antigen may cross-react with those of other tropical helminths. Due to late seroconversion, these tests may not be positive until a minimum of 6 weeks after the primary infection [2]. Although less robust, circulating cathodic antigens may be detected in the urine or serum samples using labeled monoclonal antibodies [34]. The PCR of parasite DNA in a urine sample (sensitivity 84 %, specificity 97 %) may prove useful in post-chemotherapy surveillance [35].
Treatment
In the last four decades, safer and more effective drugs have replaced the toxic older generation of anti-infective agents [36]. According to the World Health Organization, only 11.5 % of the 243 million people in 52 countries who required treatment received pharmacological intervention in 2011 [1]. Praziquantel (PZQ), an acylated quinoline–pyrazine compound, is regarded as the gold standard of therapy. It is effective against the three principal schistosomal species. With a single oral dose of 40 mg/kg body weight, it is the drug of choice for mass treatment programs [2, 37]. In the clinic setting, children are treated with two doses of 20 mg/kg PZQ at intervals ranging from 1 day to 4 weeks. Although pharmacokinetic studies are lacking, a suspension formulation or crushed tablets have been successfully used in the treatment of younger children [37, 38]. Side effects may include nausea, emesis, diarrhea, dizziness, headache and pyrexia, but these are less likely to occur with the two-daily dosing regimen than with the single-dose treatment [38]. Because first pass metabolism is mediated by the cytochrome P450 (CYP) system (e.g. CYP3A4), PZQ may interfere with the hepatic clearance of tacrolimus, cyclosporine and sirolimus [39]. Furthermore, the anti-parasitic effect may be impaired by the concurrent use of intestinal P-glycoprotein inhibitor (e.g. chloroquine) and CYP inducers (e.g. rifampin) [39, 40]. Serum level may be increased by cytochrome P450 inhibitors, such as ketoconazole and grapefruit juice [39]. To avoid worsening of the hypersensitivity reaction from the release of egg antigens by the killing of the adult worm, treatment with PZQ should be delayed in patients with Katayama fever. Instead, the initial wave of immune-related symptoms are relieved by the administration of intravenous hydrocortisone [18, 19].
Prevention
With the introduction of PZQ, mass treatment programs of high-risk populations have had a profound short-term benefit on morbidity [41, 42]. Prevention of parasite re-infection may require many years of regular annual chemotherapy. An integrated approach that includes provision of basic sanitation, clean water supply and public health education holds the greatest prospect for a more durable outcome. These measures are generally cost effective and are able to curtail other neglected tropical diseases. Development of an effective vaccine has the potential for making a considerable impact. A chimeric vaccine fusion of the antigens for both hookworm and Schistosoma parasites (Sm-TSP-2 and Na-APR-1) was recently used successfully in an experimental murine challenge study [43]. This vaccine is particularly suitable for use in areas with endemicity for both diseases. Other candidate vaccines awaiting phase I human trials are the derivatives of Sm-p80 (calpain) and Sm-14 antigens [44].
Questions (answers are provided following the Reference list)
-
1.
The following contributes to the epidemiological pattern of schistosomiasis except:
-
a.
Extreme poverty
-
b.
Dam construction
-
c.
Type of molluscs
-
d.
Tourism
-
e.
Cold climate
-
a.
-
2.
All are reasons for greater incidence of urogenital schistosomiasis in children except:
-
a.
Recreational activity
-
b.
Poor education
-
c.
Higher serum level of IgM
-
d.
Urinary tract infection
-
a.
-
3.
Urogenital schistosomiasis is a potent mediator of transitional cell carcinoma of the bladder:
-
a.
True
-
b.
False
-
a.
-
4.
Katayama fever is due to one of the following:
-
a.
Cercaria skin penetration
-
b.
Schistosomule migration
-
c.
Miracidium in the bladder wall
-
d.
Hypersensitivity to egg antigen
-
a.
-
5.
One of the following is a fairly reliable means of detecting active bladder infection:
-
a.
Urine microscopy
-
b.
Confocal laser scanning microscopy
-
c.
Ultrasonography
-
d.
Cystoscopy
-
a.
-
6.
An effective preventive strategy for endemic urinary schistosomiasis should include:
-
a.
Adequate treatment of bacterial cystitis
-
b.
Mass chemotherapy
-
c.
Health education
-
d.
Water supply
-
e.
Relocation from endemic zone
-
I.
a and c
-
ii.
a and b
-
iii.
b, c and d
-
iv.
a and e
-
I.
-
a.
-
7.
A true statement about schistosomal glomerulopathy:
-
a.
The most common pathogen is S. hematobium
-
b.
Histological type III is due to Salmonella superinfection
-
c.
Granulomatous egg deposit in renal tissue is common
-
d.
Not seen in the absence of bladder infestation
-
e.
Low serum C3 is common
-
I.
a and b
-
ii.
a and e
-
iii.
c only
-
iv.
None of the above
-
I.
-
a.
References
World Health Organization (2013) Schistosomiasis. Fact sheet no. 115. World Health Organization, Geneva, Switzerland. Available at: http://www.who.int/mediacentre/factsheets/fs115/en/
Gryseels B, Polman K, Clerinx J, Kestens L (2006) Human schistosomiasis. Lancet 368(9541):1106–1118
Steinmann P, Keiser J, Bos R, Tanner M, Utzinger J (2006) Schistosomiasis and water resources development: systematic review, meta-analysis, and estimates of people at risk. Lancet Infect Dis 6(7):411–425
Stothard JR, Sousa-Figueiredo JC, Betson M, Bustinduy A, Reinhard-Rupp J (2013) Schistosomiasis in African infants and preschool children: let them now be treated! Trends Parasitol 29(4):197–205
Ganley-Leal LM, Mwinzi PN, Cetre-Sossah CB, Andove J, Hightower AW, Karanja DM, Colley DG, Secor WE (2006) Correlation between eosinophils and protection against reinfection with Schistosoma mansoni and the effect of human immunodeficiency virus type 1 coinfection in humans. Infect Immun 74(4):2169–2176
Naus CWA, van Dam GV, Kremsner PG, Krijger FW, Deelder AM (1998) Human IgE, IgG subclass, and IgM responses to worm and egg antigens in Schistosomiasis haematobium: A 12-month study of reinfection in Cameroonian children. Clin Infect Dis 26:1142–1147
Miller P, Wilson RA (1980) Migration of the schistosomula of Schistosoma mansoni from the lungs to the hepatic portal system. Parasitology 80(2):267–288
Schroeder H, Skelly P, Zipfel PF, Losson B, Vanderplasschen A (2009) Subversion of complement by hematophagous parasites. Dev Comp Immunol 33(1):5–13
Cheever AW, Hoffmann KF, Wynn TA (2000) Immunopathology of Schistosomiasis mansoni in mice and men. Immunol Today 21:465–466
Wilson MS, Mentink-Kane MM, Pesce JT, Ramalingam TR, Thompson R, Wynn TA (2007) Immunopathology of schistosomiasis. Immunol Cell Biol 85(2):148–154
Ouf EA, Ojurongbe O, Akindele AA, Sina-Agbaje OR, Van Tong H, Adeyeba AO, Kremsner PG, Kun JF, Velavan T (2012) Ficolin-2 levels and FCN2 genetic polymorphisms as a susceptibility factor in schistosomiasis. J Infect Dis 206(4):562–570
Nicolls DJ, Weld LH, Schwartz E, Reed C, von Sonnenburg F, Freedman DO, Kozarsky PE (2008) Characteristics of Schistosomiasis in travelers reported to the GeoSentinel Surveillance Network 1997–2008. Am J Trop Med Hyg 79(5):729–734
Appleton CC (1984) Schistosome dermatitis: an unrecognized problem in South Africa? S Afr Med J 65:467–469
Jones MK, McManus DP, Sivadorai P, Glanfield A, Moertel L, Belli SI, Gobert GN (2007) Tracking the fate of iron in early development of human blood flukes. Int J Biochem Cell Biol 39(9):1646–1658
Ayoya MA, Spiekermann-Brouwer GM, Stoltzfus RJ, Nemeth E, Habicht JP, Ganz T, Rawat R, Traoré AK, Garza C (2010) α1-Acid glycoprotein, hepcidin, C-reactive protein, and serum ferritin are correlated in anemic schoolchildren with Schistosoma haematobium. Am J Clin Nutr 91(6):1784–1790
Khalaf I, Shokeir A, Shalaby M (2012) Urologic complications of genitourinary schistosomiasis. World J Urol 30(1):31–38
Hegertun IE, Sulheim Gundersen KM, Kleppa E, Zulu SG, Gundersen SG, Taylor M, Kvalsvig JD, Kjetland EF (2013) S. haematobium as a common cause of genital morbidity in girls: A cross-sectional study of children in South Africa. PLoS Negl Trop 7(3):e2104
Bottieau E, Clerinx J, de Vega MR, Van den Enden E, Colebunders R, Van Esbroeck M, Vervoort T, Van Gompel A, Van den Ende J (2006) Imported Katayama fever: clinical and biological features at presentation and during treatment. J Infect 52:339–345
Carod-Artal FJ (2008) Neurological complications of Schistosoma infection. Trans R Soc Trop Med Hyg 102:107–116
Dobardzic AM, Dobardzic R (1997) Epidemiological features of complicated UTI in a district hospital of Kuwait. Eur J Epidemiol 13(4):465–470
Nmorsi OP, Kwandu UN, Ebiaguanye LM (2007) Schistosoma haematobium and urinary tract pathogens co-infections in a rural community of Edo State, Nigeria. J Commun Dis 39(2):85–90
Barnhill AE, Novozhilova E, Day TA, Carlson SA (2011) Schistosoma-associated Salmonella resist antibiotics via specific fimbrial attachments to the flatworm. Parasit Vectors 4:123
Fedewa SA, Soliman AS, Ismail K, Hablas A, Seifeldin IA, Ramadan M, Omar HG, Nriagu J, Wilson ML (2009) Incidence analyses of bladder cancer in the Nile delta region of Egypt. Cancer Epidemiol 33(3–4):176–181
Felix AS, Soliman AS, Khaled H, Zaghloul MS, Banerjee M, El-Baradie M, El-Kalawy M, Abd-Elsayed AA, Ismail K, Hablas A, Seifeldin IA, Ramadan M, Wilson ML (2008) The changing patterns of bladder cancer in Egypt over the past 26 years. Cancer Causes Control 19(4):421–429
Seck SM, Sarr ML, Dial MC, Ka EF (2011) Schistosoma hematobium-associated glomerulopathy. Indian J Nephrol 21(3):201–203
dos-Santos WL, Sweet GM, Bahiense-Oliveira M, Rocha PN (2011) Schistosomal glomerulopathy and changes in the distribution of histological patterns of glomerular diseases in Bahia, Brazil. Mem Inst Oswaldo Cruz 106(7):901–904
Sobh M, Moustafa F, El Arbagy A, El Din MS, Shamaa S, Amer G (1990) Nephropathy in asymptomatic patients with active Schistosoma mansoni infection. Int Urol Nephrol 22:37–43
Madwar MA, O'Shea JM, Skelton JA, Soothill JF (1978) Complement components and immunoglobulins in patients with schistosomiasis. Clin Exp Immunol 34:354–358
Feldmeier H, Poggensee G (1993) Diagnostic techniques in Schistosomiasis control: a review. Acta Trop 52:205–220
Leutscher PD, Reimert CM, Vennervald BJ, Ravaoalimalala VE, Ramarokoto CE, Serieye J, Raobelison A, Rasendramino M, Christensen NO, Esterre P (2000) Morbidity assessment in urinary schistosomiasis infection through ultrasonography and measurement of eosinophil cationic protein (ECP) in urine. Trop Med Int Health 5:88–93
Bichler KH, Savatovsky I (2006) EAU guidelines for the management of urogenital schistosomiasis. Eur Urol 49(6):939–1152
Fritzsche C, Stachs O, Holtfreter MC, Nohr-Łuczak C, Guthoff RF, Reisinger EC (2012) Confocal laser scanning microscopy, a new in vivo diagnostic tool for Schistosomiasis. PLoS One 7(4):e34869
Kinkel HF, Dittrich S, Bäumer B, Weitzel T (2012) Evaluation of eight serological tests for diagnosis of imported schistosomiasis. Clin Vaccine Immunol 19(6):948–953
Tchuem Tchuenté L-A, Kueté Fouodo CJ, Kamwa Ngassam RI, Sumo L, Dongmo Noumedem C, Kenfack CM, Gipwe NF, Nana ED, Stothard JR, Rollinson D (2012) Evaluation of circulating cathodic ntigen (CCA) urine—tests for diagnosis of Schistosoma mansoni infection in Cameroon. PLoS Negl Trop Dis 6(7):e1758
Ibironke OA, Phillips AE, Garba A, Lamine SM, Shiff C (2011) Diagnosis of Schistosoma haematobium by detection of specific DNA fragments from filtered urine samples. Am J Trop Med Hyg 84(6):998–1001
Fenwick A, Savioli L, Engels D, Robert BN, Todd MH (2003) Drugs for the control of parasitic diseases: current status and development in schistosomiasis. Trends Parasitol 19:509–515
Erko B, Degarege A, Tadesse K, Mathiwos A, Legesse M (2012) Efficacy and side effects of praziquantel in the treatment of Schistosomiasis mansoni in schoolchildren in Shesha Kekele Elementary School, Wondo Genet, Southern Ethiopia. Asian Pac J Trop Biomed 2(3):235–239
Stothard JR, Sousa-Figueiredo JC, Betson M, Green HK, Seto EY, Garba A, Sacko M, Mutapi F, Vaz Nery S, Amin MA, Mutumba-Nakalembe M, Navaratnam A, Fenwick A, Kabatereine NB, Gabrielli AF, Montresor A (2011) Closing the praziquantel treatment gap: new steps in epidemiological monitoring and control of schistosomiasis in African infants and preschool-aged children. Parasitology 138(12):1593–1606
Castro N, Jung H, Medina R, González-Esquivel D, Lopez M, Sotelo J (2002) Interaction between grapefruit juice and praziquantel in humans. Antimicrob Agents Chemother 46(5):1614–1616
Hayeshi R, Masimirembwa C, Mukanganyama S, Ungell AL (2006) The potential inhibitory effect of antiparasitic drugs and natural products on P-glycoprotein mediated efflux. Eur J Pharm Sci 29(1):70–81
Prichard RK, Basáñez MG, Boatin BA, McCarthy JS, García HH, Yang GJ, Sripa B, Lustigman S (2012) A research agenda for Helminth diseases of humans: intervention for control and elimination. PLoS Negl Trop Dis 6(4):e1549
King CH, Olbrych SK, Soon M, Singer ME, Carter J (2011) Colley DG (2011) Utility of repeated praziquantel dosing in the treatment of schistosomiasis in high-risk communities in Africa: a systematic review. PLoS Negl Trop Dis 5(9):e1321
Pearson MS, Pickering DA, McSorley HJ, Bethony JM, Tribolet L, Dougall AM, Hotez PJ, Loukas A (2012) Enhanced protective efficacy of a chimeric form of the schistosomiasis vaccine antigen Sm-TSP-2. PLoS Negl Trop Dis 6(3):e1564
Ahmad G, Zhang W, Torben W, Ahrorov A, Damian RT, Wolf RF, White GL, Carey DW, Mwinzi PN, Ganley-Leal L, Kennedy RC, Siddiqui AA (2011) Preclinical prophylactic efficacy testing of Sm-p80-based vaccine in a nonhuman primate model of Schistosoma mansoni infection and immunoglobulin G and E responses to Sm-p80 in human serum samples from an area where schistosomiasis is endemic. J Infect Dis 204(9):1437–1444
Author information
Authors and Affiliations
Corresponding author
Additional information
Answers:
1. e
2. d
3. b
4. d
5. b
6. iii
7. iv
Rights and permissions
About this article
Cite this article
Bamgbola, O.F. Urinary schistosomiasis. Pediatr Nephrol 29, 2113–2120 (2014). https://doi.org/10.1007/s00467-013-2723-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-013-2723-1