Abstract
Background
ABO-incompatible renal transplantation (ABOi-RTx) following preconditioning with immunoadsorption (IA) and rituximab is a promising approach to facilitate living-related RTx. However, clinical experience is limited in pediatric patients.
Methods
Three patients underwent living-related ABOi-RTx in our center. Preoperative IA was performed six, ten and 11 times in patient one, two and three, respectively, to achieve isoagglutinin titers of ≤1:8 on the day of transplantation; rituximab was administered once. The immunosuppressive regimen further comprised tacrolimus, mycophenolate, methylprednisolone and basiliximab; immunoglobulin G (IgG) was infused on the day of ABOi-RTx.
Results
All three patients achieved normal renal function within 2–6 days post-RTx. Major postoperative bleeding occurred in two patients, with one requiring repeated blood transfusions and the other a surgical revision 4 h after RTx, despite local citrate anticoagulation use during the preoperative IA procedures in the latter patient. A pyelonephritis-associated increase of the isoagglutinin IgG/IgM titers to 1:64/1:128 led to a biopsy-proven acute humoral rejection in the third patient, which was treated successfully with plasma exchange and methylprednisolone pulses. The estimated glomerular filtration rate at 18, 8 and 23 months post-RTx was 96, 52 and 74 ml/min/1.73 m2, respectively.
Conclusions
ABOi-RTx can successfully be performed in pediatric patients after preconditioning with quadruple immunosuppression, rituximab and IA. Caution is required regarding bleeding complications, which are most likely due to the unspecific binding of coagulation factors during repeated IA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
ABO blood group antigens are expressed by the endothelium of solid organs, including the kidney; sensitization leads to hyperacute antibody-mediated allograft rejection. ABO-incompatibility (ABOi) has therefore been considered as a major barrier to renal transplantation (RTx) in the past. Depending on blood group distribution in different populations, until recently up to one-third of potential kidney donors had to be excluded from living donation due to ABO incompatibility or had to undergo aggressive preconditioning protocols to overcome the immunological barrier and to enlarge the pool of potential living donation [1]. These protocols included intensified plasma exchange (PE), splenectomy, antilymphocyte antibodies and cyclophosphamide and yielded satisfactory graft and patient survival rates similar to blood group compatible (ABOc) RTx [2–4].
The introduction of rituximab, a CD20 antibody which eliminates CD20 positive B-cells in the peripheral blood, in combination with repeated, isoagglutinin titer-guided, preoperative immunoadsorption (IA) has enabled a less aggressive preconditioning approach to be used that is devoid of splenectomy and antilymphocyte globulin. Outcome rates have been excellent with 12- to 18-month graft survival rates of 91–97 % [5–8] and patient survival rates of 97–100 % [5, 6]. In one post-transplant patient cohort, after 4.5 years, patient survival was 93 % and graft survival was 91 % [7].
Clinical studies on the pediatric patient population are less numerous. To date, one successful pediatric case [9] and ten children from one center, undergoing ABOi-RTx with rituximab and IA [10], have been reported. Seven of the ten children in the latter study required IA, which were performed with the Glycosorb® column (Glycorex Transplantation AB, Lund, Sweden), specifically binding blood group isoagglutinins. In these ten children, RTx was successful, the postoperative course was uneventful and graft function was comparable with pediatric ABOc-RTx after 1–3 years [10].
Here, we report on three children with successful ABOi-RTx following preconditioning with IA and rituximab, of whom two experienced major bleeding complications that were possibly related to the unspecific binding of coagulation factors during extended IA treatment.
Subjects and methods
Since 2009 three patients aged 1.9, 18.2 and 19.7 years have undergone ABOi-RTx in our center; the underlying renal diseases were congenital nephrotic syndrome, obstructive uropathy and nephronophthisis, respectively. The patient with obstructive uropathy underwent bladder augmentation and appendix stoma creation 6 weeks prior to RTx. Blood groups were A Rh+, A Rh+ and B Rh− in patients 1, 2 and 3, respectively, and B Rh+, B Rh+ and A Rh− in the respective donating parents. The number of HLA-mismatches were 3, 3 and zero, respectively. In the three patients, preconditioning comprised a single dose of rituximab (375 mg/m2) 24, 1 and 44 days before RTx, respectively, and repeated IA according to isoagglutinin titers. The pharmacological immunosuppression regimen consisted of methylprednisolone, tacrolimus and mycophenolate mofetil (MMF) and was initiated with the first IA; target trough levels were 10–12 μg/L and 1.5–4 mg/L for tacrolimus and MMF, respectively. The interleukin-2 receptor (CD25) antibody basiliximab was given prior to and 4 days after RTx [10 mg when the body weight (b.w.) was <35 kg; 20 when the b.w. was >35 kg]. Intravenous immunoglobulin G (IgG; 0.5 g/kg b.w.) was administered on the day of RTx. All three patients received cytomegalovirus (CMV) prophylaxis with valganciclovir for 100 days, and patient one received CMV hyperimmune globulin. Pneumocystis jiroveci prophylaxis with cotrimoxazole was prescribed for all patients for 6 months.
IA was performed using Globaffin® (Fresenius Medical Care, Bad Homburg, Germany), Immunosorba® (Fresenius Hemocare, St. Wendel, Saarland, Germany) and Glycosorb® (Glycorex Transplantation AB, Lund, Sweden) columns in patient one to three, respectively; the adsorber surface area to body surface area ratio was 0.59, 0.42 and 0.41, respectively. The number of IA sessions in patients one, two and three were six, ten and 11, and the plasma turn-over target was 2,000 ml/m2 per session (equal to 1.5 fold the plasma volume), which could be achieved in all three children (2,068 ± 92 mL/m2) within 1–2.5 h. Total plasma turn-over was 12,020, 21,335 and 22,647 ml/m2 in patient one, two and three, respectively. Isoagglutinin IgG and IgM antibody titers were determined by the indirect Coombs test (IgG) and the saline method (IgM), respectively; targets were ≤1:4 post-IA and ≤1:8 on the day of transplant surgery. Coagulation parameters were repeatedly monitored pre-IA and at least once within 4 h post-IA in each patient. Glomerular filtration rate was estimated (eGFR) using the new Schwartz formula [11] in patient 1 and the Modification of Diet in Renal Disease (MDRD) [12] formula in patients two and three. The tacrolimus area under the concentration–time curve (AUC) was calculated from an abbreviated pharmacokinetic profile consisting of blood level measurements before dosing and 1, 2 and 4 h after dosing [13].
Results
Preoperative IA was performed six, ten and 11 times in patient one, two and three, respectively. On the day of ABOi-RTx, the IgG and IgM isoagglutinin titer were 1:2/0, 0/0 and 1:8/1:4, respectively. During the IA procedures, anticoagulation consisted of heparin in patient one and two and of citrate in patient three. Local citrate anticoagulation was also used in patient one on the day prior to RTx.
Median activated clotting time (ACT) during IA was 325 s (range 200–576 s) in patient one and 143 s (100–338 s) in patient two. Following IA, the activated partial thromboplastin time (aPTT) and international normalized ratio (INR) were markedly increased, and the serum fibrinogen level decreased and remained so in patient two and three on the day of RTx (Table 1). Coagulation values were not repeated on the day of ABOi-RTx, and none of the children received any blood products prior to surgery. In the 25 children undergoing ABOc-RTx in our center within the last 17 months, all pre- and postoperative aPTT, INR and fibrinogen values were within the normal range.
No anticoagulation was given during transplant surgery. Postoperative anticoagulation consisted of 100 IU heparin/kg b.w. per day in patient one, started 1.5 h post-RTx until day 7. Patient two received a total dose of heparin of 10 IU/kg b.w. during postoperative hours 4–6, following which time abdominal bleeding was diagnosed, requiring the transfusion of 3 U of fresh frozen plasma and 3 U of packed erythrocytes. Patients three, who was treated with the isoagglutinin-specific Glycosorb® column, did not receive any anticoagulation but developed major bleeding which required the transfusion of 4 U of packed erythrocytes and surgical revision 4 h after the initial operation, which revealed diffuse bleeding. The transfusion of fresh frozen plasma normalized the aPPT and fibrinogen and improved INR in both patients; platelets were not given (Table 1).
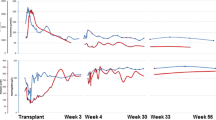
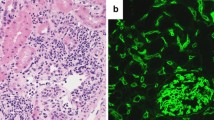
The three transplantations were successful, and serum creatinine normalized within 2, 4, and 6 days in the respective patient. Isoagglutinin antibody titers remained low postoperatively, except for patient two (Fig. 1) who experienced an increase in anti-B IgG/IgM titers to 1:64/1:128 on day 9, in association with pyelonephritis. This dramatic rise of the isoagglutinin titers led to biopsy-proven, acute antibody-mediated rejection (Banff type II), which was treated successfully by PE and methylprednisolone pulse therapy. Two subsequent pyelonephritis episodes were not associated with major increases in isoagglutinin titer (Fig. 1b). No further bacterial or viral infections were reported in the three patients over an observation period of 16 (range 8–23) months. Tacrolimus trough levels were 4.8–18.2 mg/L; the TAC-AUC determined during the third week post-RTx was 114, 223 and 259 μg*h/L, respectively. The further clinical course of these three patients was unremarkable; the eGFR values at 18, 8 and 23 months post-RTx were 96, 52 and 74 ml/min per 1.73 m2, respectively.
Pre- and postoperative course of immunoglobulin G (IgG) and IgM blood group antibody titers and complications in three children who underwent ABO incompatible renal transplantation (ABOi-RTx). The children underwent preconditioning with rituximab and repeated immunoadsorption (IA). IA resulted in a decline in isoagglutinins by not more than one titer per session and was therefore repeated 6, 10 and 11 times (arrows), in patient 1, 2 and 3, respectively. Patient 2 (central graph) and patient 3 (lower graph) developed major bleeding episodes (solid inverted triangle). Patient 2 further developed a marked increase in isoagglutinin titers after ABOi-RTx and a biopsy-proven acute antibody-mediated rejection (hatch) in association with transplant pyelonephritis episodes (asterisk)
Discussion
Preconditioning of ABOi-RTx recipients with rituximab and IA has successfully been introduced in adult patients with CKD stage 5 [14]. However, corresponding pediatric experience is limited to a few centers of which only two have reported their experience in the form of a single case report [9] and a series of ten children [10], respectively. All patients had a favorable outcome devoid of complications. We report here on three children who successfully underwent ABOi-RTx, although two subsequently suffered from major bleeding complications.
The patients treated in our center required more IA sessions than the pediatric patients reported previously [9, 10]. The IA treatment was associated with a transient, marked increase in aPTT and INR and a 40 % decline in plasma fibrinogen concentrations, even when the ABO isoagglutinin-specific Glycosorb® column was used. After the ABOi-RTx sessions, the coagulation parameters were still out of the normal range in two of the three patients, although they normalized after they received transfusions of fresh frozen plasma. In a previous series of adult ABOi-RTx, three of 17 patients developed major bleeding complications, one following IA with the Glycosorb® column [15]. Renner et al. reported diffuse postoperative bleeding complications in one-third of 14 ABOi-RTx adult patients treated with IA [16]. These were found to be related to the intraoperative flushing of the allograft with 10,000–20,000 IU heparin, and a reduction of the dose of heparin to 4,000 IU resulted in lower post-RTx aPTT values and no further bleeding episodes. Wilpert et al. reported immediate postoperative bleeding episodes in ten of 40 patients undergoing six (range 1–17) IA sessions with the Glycosorb® column prior to ABOi-RTx, compared to five bleeding episodes in 43 patients with ABOc-RTx [17]. Habicht et al. reported a 4.5-fold increased incidence of hemorrhagic complications in 21 ABOi-RTx patients, as compared to ABOc-RTx patients, who underwent two to six preoperative IA sessions using the Glycosorb® column [8]. Of the ten children transplanted in the Stockholm case series [10], only seven required IA, performed with the Glycosorb® column, of whom six required four IA sessions only; none developed hemorrhagic complications. The two children with bleeding complication in our center did not receive intraoperative heparin or significant amounts of heparin postoperatively. They underwent 10 and 11 IA sessions within 45 and 16 days, respectively. The latter patient was treated with the Glycosorb® column using only local citrate anticoagulation. A potential explanation of the high bleeding risk associated with IA is the efficient binding of not only isoagglutinins, but also coagulation factors. Whereas the manufacturer of this column does not warm of the possible binding of coagulation factors, previous studies have reported a decline in fibrinogen plasma levels with repeated IA sessions [18, 19] and the presence of numerous proteins involved in the coagulation process in IA eluates [20].
The high number of IA sessions required was not unexpected and is in line with the extended experience in adults. Two adult patients reported in the literature were unable to undergo ABOi-RTx despite rituximab infusion and 13 and 18 IA and PE sessions, respectively [8, 21]. Of note, only four of the 74 pediatric patients who underwent ABOc-RTx in our center during the same time 3-year period developed bleeding complications that required red blood cell transfusions; three patients had received heparin (100–200 IU/kg per day) and one patient Orgaran (danaparoid sodium). Even though the number of patients in our study is limited to three, our findings argue in favor of a close monitoring of global coagulation parameters in the case of ABOi-RTx and repeated IA sessions. In the case of compromised coagulation on the day of ABOi-RTx, infusion of prothrombin complex concentrates or fresh frozen plasma from ABO-compatible donors may be considered prior to surgery.
Acute antibody-mediated rejections due to an increase in isoagglutinin titers were frequently observed prior to the clinical use of rituximab and IA in adults and children [3, 4, 21], but they have since become rare events. The 11 children with rituximab and IA preconditioning thus far reported in the literature did not experience humoral rejection or postoperative infections [9, 10]. We observed one case with a pyelonephritis-associated marked increase of isoagglutinin titers that led to acute antibody-mediated rejection. This secondary increase of isoagglutinin titers triggered by a systemic infection and resulting in an acute antibody-mediated rejection has not been reported previously. Our successful treatment with PE and glucocorticoid pulse therapy is in line with the reported outcome in adults [22].
In conclusion, ABOi-RTx can successfully be performed in pediatric patients after preconditioning with rituximab and IA and quadruple immunosuppressive therapy. Caution is required regarding an increased risk of bleeding complications, most likely due to the unspecific binding of coagulation factors during IA.
References
Beimler J, Zeier M (2007) ABO-incompatible transplantation–a safe way to perform renal transplantation? Nephrol Dial Transplant 22:25–27
Takahashi K, Saito K, Takahara S, Okuyama A, Tanabe K, Toma H, Uchida K, Hasegawa A, Yoshimura N, Kamiryo Y, Japanese ABO-Incompatible Kidney Transplantation Committee (2004) Excellent long-term outcome of ABO-incompatible living donor kidney transplantation in Japan. Am J Transplant 4:1089–1096
Shishido S, Asanuma H, Tajima E, Hoshinaga K, Ogawa O, Hasegawa A, Honda M, Nakai H (2001) ABO-incompatible living-donor kidney transplantation in children. Transplantation 72:1037–1042
Ohta T, Kawaguchi H, Hattori M, Takahashi K, Nagafuchi H, Akioka Y, Mizushima W, Ishikawa N, Tanabe K, Toma H, Takahashi K, Ota K, Ito K (2000) ABO-incompatible pediatric kidney transplantation in a single-center trial. Pediatr Nephrol 14:1–5
Tydén G, Donauer J, Wadström J, Kumlien G, Wilpert J, Nilsson T, Genberg H, Pisarski P, Tufveson G (2007) Implementation of a Protocol for ABO-incompatible kidney transplantation—a three-center experience with 60 consecutive transplantations. Transplantation 83:1153–1155
Kahwaji J, Sinha A, Toyoda M, Ge S, Reinsmoen N, Cao K, Lai CH, Villicana R, Peng A, Jordan S, Vo A (2011) Infectious complications in kidney-transplant recipients desensitized with rituximab and intravenous immunoglobulin. Clin J Am Soc Nephrol 6:2894–2900
Genberg H, Kumlien G, Wennberg L, Tyden G (2011) The efficacy of antigen-specific immunoadsorption and rebound of anti-A/B antibodies in ABO-incompatible kidney transplantation. Nephrol Dial Transplant 26:2394–2400
Habicht A, Bröker V, Blume C, Lorenzen J, Schiffer M, Richter N, Klempnauer J, Haller H, Lehner F, Schwarz A (2011) Increase of infectious complications in ABO-incompatible kidney transplant recipients–a single centre experience. Nephrol Dial Transplant 26:4124–4131
Ahlenstiel T, Offner G, Strehlau J, Pape L, Froede K, Ehrich JH, Schwarz A, Heuft HG, Klempnauer J (2006) ABO-incompatible kidney transplantation of an 8-yr-old girl with donor/recipient-constellation A1B/B. Xenotransplantation 13:141–147
Tydén G, Kumlien G, Berg UB (2011) ABO-incompatible kidney transplantation in children. Pediatr Transplant 5:502–504
Schwartz GJ, Muñoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL (2009) New equations to estimate GFR in children with CKD. J Am Soc Nephrol 20:629–637
Crowe E, Halpin D, Stevens P, Guideline Development Group (2008) Early identification and management of chronic kidney disease: summary of NICE guidance. Br Med J 337:1530
Filler G, Feber J, Lepage N, Weiler G, Mai I (2002) Universal approach to pharmacokinetic monitoring of immunosuppressive agents in children. Pediatr Transplant 6:411–418
Tydén G, Kumlien G, Fehrman I (2003) Successful ABO-incompatible kidney transplantations without splenectomy using antigen-specific immunoadsorption and rituximab. Transplantation 76:730–731
Morath C, Becker LE, Leo A, Beimler J, Klein K, Seckinger J, Kihm LP, Schemmer P, Macher-Goeppinger S, Wahrmann M, Böhmig GA, Opelz G, Süsal C, Zeier M, Schwenger V (2012) ABO-incompatible kidney transplantation enabled by non-antigen-specific immunoadsorption. Transplantation 93:827–834
Renner FC, Czekalinska B, Kemkes-Matthes B, Feustel A, Stertmann WA, Padberg W, Weimer R (2010) Postoperative bleeding after AB0-incompatible living donor kidney transplantation. Transplant Proc 42:4164–4166
Wilpert J, Fischer KG, Pisarski P, Wiech T, Daskalakis M, Ziegler A, Neumann-Haefelin E, Drognitz O, Emmerich F, Walz G, Geyer M (2010) Long-term outcome of ABO-incompatible living donor kidney transplantation based on antigen-specific desensitization. An observational comparative analysis. Nephrol Dial Transplant 25:3778–3786
Gjörstrup P, Watt RM (1990) Therapeutic protein a immunoadsorption. A review. Transfus Sci 11:281–302
Hosokawa S, Oyamaguchi A, Yoshida O (1990) Clinical studies on adequate dosage of heparin during lmmunoadsorption with membrane plasmapheresis. J Clin Apher 5:197–200
Kienbaum M, Koy C, Montgomery HV, Drynda S, Lorenz P, Illges H, Tanaka K, Kekow J, Guthke R, Thiesen HJ, Glocker MO (2009) MS characterization of apheresis samples from rheumatoid arthritis patients for the improvement of immunoadsorption therapy—a pilot study. Proteomics Clin Appl 3:797–809
Takahashi K (2001) ABO-incompatible kidney transplantation. Elsevier Science BV, Amsterdam, pp 1–154
Biglarnia AR, Nilsson B, Nilsson Ekdahl K, Tufveson G, Nilsson T, Larsson E, Wadström J (2012) Desensitization with antigen-specific immunoadsorption interferes with complement in ABO-incompatible kidney transplantation. Transplantation 93:87–92
Acknowledgments
We thank the nurses of the pediatric dialysis unit for their dedicated care of the patients and continuous support with data collection.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schaefer, B., Tönshoff, B., Schmidt, J. et al. Bleeding complications in pediatric ABO-incompatible kidney transplantation. Pediatr Nephrol 28, 327–332 (2013). https://doi.org/10.1007/s00467-012-2302-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-012-2302-x