Abstract
Background
Urinary interleukin-18 and cystatin-C are suggested to be biomarkers for predicting acute kidney injury (AKI). The aims of this study are to examine whether the urinary concentrations of interleukin-18 and cystatin-C vary with gestational age and other factors in non-AKI control neonates, and to determine whether urinary interleukin-18 and cystatin-C can predict AKI development in non-septic critically ill neonates, independently of potential confounders.
Methods
We enrolled 62 non-septic critically ill neonates. Urine was collected every 48–72 h during the first 10 days of life.
Results
Urinary concentration of cystatin-C, but not interleukin-18, decreased with increasing gestational age and body weight, but not with increasing postnatal age in non-AKI control neonates. Both urinary interleukin-18 and cystatin-C were associated with AKI, even after controlling for gestational and postnatal age, birth weight, gender, Apgar score and the score for neonatal acute physiology in non-septic critically ill neonates. Urinary interleukin-18 and cystatin-C had odds ratios of 2.27 and 2.07, and achieved the area under-the-receiver-operating-characteristic curve of 0.72 and 0.92, respectively, for predicting AKI.
Conclusions
The urinary concentration of cystatin-C, but not interleukin-18, may decrease with increasing renal maturity. Both urinary interleukin-18 and cystatin-C are independently predictive of AKI in non-septic critically ill neonates.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute kidney injury (AKI) is an independent risk factor for mortality [1]. Critically ill neonates are at a high risk of having AKI, as they are commonly exposed to perinatal anoxia/hypoxia and nephrotoxic medications and have frequent infections that lead to multi-organ failure [2, 3]. The estimated reported incidence of AKI in critically ill neonates ranges from 8 to 24% and the mortality rate is between 10 and 61% [4]. The exact incidence of AKI in neonates, however, is unknown, because there is a lack of a classification system to define AKI in the neonatal population [3]. Efforts to define and characterize AKI in neonates, leading to the detection of early AKI, will ultimately contribute to an improvement in neonatal outcome.
Serum creatinine (Cr) is the most commonly used clinical measure of renal function, but it is a poor marker for diagnosis of AKI. The use of serum Cr in neonates, especially in premature neonates, is further complicated by maturational change during postnatal renal development [3]. There is a very wide distribution of normal serum Cr levels in neonates. Serum Cr reflects maternal levels during the first 2 days of life. In full-term healthy neonates, the glomerular filtration rate (GFR) rapidly increases and the serum Cr declines progressively with increasing postnatal age to reach a stable neonatal level by 2 weeks of life [5, 6]. Serum Cr, however, does not fall steadily from birth in very preterm neonates. In neonates with less than 26 weeks of gestational age, there is a transient increase in serum Cr, reaching a peak on day 3 of life, followed by a progressive decline [6, 7].
Recent research in AKI has focused on identifying biomarkers, characterized as early, noninvasive, and sensitive indicators of AKI. The early detection of AKI could optimize and improve patient outcomes [8–10]. Two of the most promising biomarkers of AKI are urinary interleukin-18 (uIL-18) [11–13] and urinary cystatin-C (uCysC) [14, 15], although there is inconsistent evidence regarding the predictive role of these biomarkers [16, 17]. Urinary IL-18 has the potential to diagnose AKI within hours of an insult. Unlike serum Cr, IL-18 is an 18-kDa pro-inflammatory cytokine that is induced in the proximal tubule after AKI, and released into urine after cleavage by caspase-1 [18–20]. Previous studies in both critically ill children and adults revealed that uIL-18 concentrations rise 24–48 h prior to AKI defined by risk, injury, failure, loss, end-stage renal disease (RIFLE) criteria [12, 21]. In critically ill children without sepsis, uIL-18 is a reliable test for early diagnosis of AKI and independently predicts the severity of AKI and mortality [21].
CysC, a 13-kDa protein, is normally filtered freely and has been proposed as a promising endogenous marker of GFR in both adults and children [22, 23]. It is considered to be completely reabsorbed and catabolized in the proximal tubule. CysC is therefore not normally found in urine in significant amounts. The appearance of increased concentrations of CysC in urine may reflect renal tubular injury and impairment [24, 25]. Studies in adults undergoing cardiothoracic surgery and in general critically ill adults demonstrate that uCysC is an early predictor of AKI and an independent predictor of mortality [14, 15].
Both uIL-18 and uCysC are detectable in neonates [26]. There is an additional challenge in the discovery of early biomarkers of AKI in this population due to the maturational change of biomarkers during renal development [3]. Two interesting clinical studies published recently have demonstrated that the baseline value of uCysC, but not uIL-18, varies by gestational age in premature infants [26]. In very low birth weight Infants, the urinary level of CysC, but not of IL-18, was associated with AKI during the early postnatal period [27]. However, the previous studies were performed in premature infants, including infants with sepsis, and it has been suggested that sepsis is significantly associated with the levels of both uIL-18 and uCysC [14, 19]. We therefore designed the present study to test the hypothesis that both uIL-18 and uCysC might predict AKI development in non-septic critically ill neonates.
The aims of the present study are (1) to examine whether the concentration of IL-18 and CysC varies with gestational and postnatal age, birth weight, body weight, gender, Apgar score, and the score for neonatal acute physiology (SNAP) in non-AKI control neonates during the first 10 days of life, (2) to determine the association between the urinary biomarkers and AKI in non-septic critically ill neonates, and (3) to evaluate whether uIL-18 and uCysC could serve as early predictors of AKI in this population, independently of potential confounders.
Patients and methods
Subject selection
All neonates admitted to the neonatal intensive care unit (NICU) on the first 3 days of life during the period from August to December 2010 were eligible for this prospective study. We excluded neonates with known congenital abnormality of the kidney, neonates with clinical diagnosis of infection, neonates undergoing exchange transfusion, and neonates with less than two serum Cr measurements. The Institutional Review Board at the Children’s Hospital affiliated to Soochow University approved the study. Informed parental consent was obtained at enrollment of each neonate.
Clinical data collection
Maternal data collected included pregnancy complication, medication, and mode of delivery. Neonatal data collected included gestational age, birth weight, Apgar score, and delivery room resuscitation course. Gestational age was calculated from the mother’s menstrual history and was confirmed by ultrasonography. SNAP was calculated on the day of NICU admission [28]. Clinical laboratory results such as serum electrolytes, Cr, blood urea nitrogen, and C-reactive protein, as well as results of renal ultrasound, lung X-ray, and echocardiogram were recorded for each neonate as part of routine care. Duration of mechanical ventilation, medications, and 28-day mortality were also recorded. Body weight, body length, total fluid administration, and urine output were recorded at sampling during the first 10 days of life.
Diagnosis of AKI
Diagnosis of AKI was based on serum Cr > 1.5 mg/dl (132.6 μmol/l) on the first 3 days of life (sustained at least 48 h, the mother of the neonate has normal renal function) and on the modified pediatric RIFLE (pRIFLE) classification after the first 3 days of life. Estimated Cr clearance (eCCl) was calculated using the Schwartz formula [29, 30]. The pRIFLE classification has been evaluated in several pediatric populations: pRIFLE R (Risk) was defined as a ≥25% decrease in eCCl; pRIFLE I (Injury) as a ≥50% decrease; and pRIFLE F (Failure) as a ≥75% decrease in eCCl from baseline renal function [21, 31, 32]. Baseline eCCl was calculated on the third day of life.
Urine sample collection
Of the total 62 neonates, 41 were admitted to the unit on the first day of life, 14 on the second day of life, and seven neonates were admitted to the unit on the third day of life. Urine samples were collected every 48–72 h during the first 10 days of life.
Measurement of urinary IL-18
After collection, urine was immediately frozen and stored at −80°C. For the measurement of IL-18, urine was centrifuged at 1,500 × g at 4°C for 10 min, and the supernatants were aliquoted and measured using a human IL-18 enzyme-linked immunosorbent assay (ELISA) kit (eBioscience BMS267/2, Vienna, Austria) that specifically detects the mature form of IL-18. The cross-reactivity of the kit for pro-IL-18 is extremely low [13, 33]. The assay was performed according to the manufacturer’s protocol. Briefly, IL-18 standards or samples were applied onto the precoated microwells. Microwells were then incubated for 2 h at room temperature and then washed with washing buffer. In succession, biotin-conjugated anti-human IL-18 monoclonal antibody and streptavidin-horseradish peroxidase were incubated in the wells for 1 h. After washing, a substrate was added for 10 min in the dark before adding stop solution. Finally, IL-18 concentration was measured at 450-nm wavelength in each well. The linearity was observed in the range of 78–5,000 pg/ml with correlation coefficient (R2) > 0.998 in this study. The detection limit for IL-18 was 12.5 pg/ml. The coefficient of variation of inter-assay and intra-assay reproducibility for IL-18 concentration ranges from 5–11%, corresponding to that reported by the kit manufacturer. The measurements were also repeated on six ‘standard’ urine samples that were run together with every set of the patient samples to confirm the reliability of IL-18 results in this study. Final uIL-18 values were expressed in picograms per milliliter (pg/ml) or picograms per milligram of urinary Cr (pg/mg uCr).
Measurement of urinary CysC
The concentration of uCysC from the same samples was measured on an automatic biochemical analyzer (Hitachi 7600, Tokyo, Japan) by immunoturbidimetry assay and expressed in nanograms per milliliter (ng/ml) or nanograms per milligram of urinary Cr (ng/mg uCr). The detection limit for CysC was 10 ng/ml. The laboratory investigators were blinded to the sample sources and clinical outcomes. In addition, urinary Cr was measured automatically by the Jaffe’s method without deproteinization.
Data management, interpretation, and analysis
The results of uIL-18 and uCysC with and without adjustment for urinary Cr were analyzed.
Determination of whether the level of uIL-18 and uCysC varies with gestational age and other factors:
The initial and the peak values of urinary biomarkers were used for association analysis. For each neonate, the value of IL-18 and CysC from the urine sample collected on the first day admitted to our unit was denoted as first uIL-18 and first uCysC. Using all samples collected, the highest uIL-18 and uCysC concentration for each neonate was denoted as peak uIL-18 and peak uCysC.
We examined the association between the first or peak value of urinary biomarkers and gestational age, birth weight, postnatal age, body weight, gender, Apgar score, or SNAP, in order to determine whether the level of uIL-18 and uCysC varies with gestational age and other factors. Since AKI may represent a confounding factor, the association among these variables was evaluated exclusively in non-AKI control neonates.
We also compared the peak concentration of uIL-18 or uCysC between AKI and non-AKI neonates.
Determination of the association between urinary biomarkers and AKI:
Using the urine samples collected 0–48 h prior to the clinical diagnosis of AKI and the first urine samples collected from neonates who did not develop AKI during the study period, we determined whether uIL-18 and uCysC would predict the development of AKI within 48 h in non-septic critically ill neonates.
We also used these urine samples to calculate the diagnostic characteristics of uIL-18 and uCysC to predict AKI development.
Statistical analysis
Statistical analyses were performed using SPSS Statistics 13.0. Assumptions of normality and homogeneity of variance were first checked. For continuous variables with a normal distribution, descriptive results were presented as a mean and a standard deviation (SD). The t test for unpaired samples was used to analyze the differences between groups. For continuous variables with a skewed distribution, descriptive results were expressed as a median and a range. The Mann–Whitney U test and the two-sample Kolmogorov–Smirnov test were used to evaluate the differences between groups. The non-parametric Friedman’s test was performed for related samples. The concentration of urinary biomarkers was log-transformed due to a skewed distribution when analysis of covariance (ANCOVA) was used to adjust for baseline differences. Categorical variables were expressed as proportions. The significance of differences between proportions or percentages was determined by the Chi-square test or Fisher's exact test when the expected value was less than 5. The relationship between urinary biomarkers and other variables was assessed by univariate and multiple linear regression analysis. Linear regression analysis was performed on log-transformed or square root-transformed data when necessary to meet the assumption of homogeneity of variances (Levene's test). Logistic regression was used to determine whether a urinary biomarker was a predictor of AKI development within 48 h, independent of potential confounders. Model fit was assessed with the Hosmer–Lemeshow goodness-of-fit test. A non-significant value for the Hosmer–Lemeshow Chi-square test suggests an absence of biased fit. A receiver operating characteristic (ROC) curve was constructed and the area under the ROC curve (AUC) was calculated to assess the predictive strength. Optimal cut-off points to maximize both sensitivity and specificity were also determined. Differences with p values <0.05 were considered to be statistically significant. All probability values are two-sided.
Results
Patient characteristics
The study involved 62 neonates. Of a total of 108 neonates admitted to our unit on the first 3 days of life during the study period, 46 were excluded: five were diagnosed with culture-proven sepsis, 31 were clinically diagnosed with sepsis, pneumonia, or TORCH syndrome, six underwent exchange transfusion, and four had less than two serum Cr measurements. Of the 62 neonates, 11 fulfilled the criteria for AKI during the first 10 days of life. Three neonates had serum Cr > 1.5 mg/dl on the first 3 days of life and sustained 48 h (the mother of the neonate has normal renal function): one on the first day and two on the third day of life. Eight neonates developed pRIFLE-I: two on the fourth day of life, one on the fifth, one on the sixth, and four on the seventh day of life. Clinical characteristics of neonates with and without AKI are shown in Table 1. None of the neonates was born from mothers with renal dysfunction or diabetes mellitus. All neonates received cephalosporins. However, none of them received aminoglycosides, vancomycin, or ibuprofen. There was a significant difference in gestational age and birth weight between AKI and non-AKI neonates. The median of Apgar score at both 1- and 5-min score was significantly lower in neonates with AKI as compared with those without AKI. The median of SNAP and the 28-day mortality was significantly higher in AKI than in non-AKI neonates.
Urinary biomarkers
A total of 178 samples of a possible 215 samples (83%) were collected: 16 neonates had four samples, 30 neonates had three samples, and eight neonates had two samples. Six neonates were discharged to home before the 10th day of life. The missing samples were because of a failure in collecting urine. Urinary IL-18 was detectable in 113 (64%) samples. Urinary CysC was detectable in 162 (91%) samples.
The results of uIL-18 and uCysC with adjustment for urinary Cr are shown in the present study. Although there was a very similar trend, the results were attenuated somewhat when the absolute value of urinary biomarkers without adjustment for urinary Cr was analyzed.
Variation of the concentration of uIL-18 and uCysC in non-AKI control neonates during the first 10 days of life
The effect of postnatal development on the level of urinary biomarkers during early life was first investigated exclusively in non-AKI control neonates.
Analysis of the data from non-AKI neonates that had four urinary samples during the first 10 days of life showed no significant difference in the level of uIL-18 or uCysC over the age period (n = 14, p > 0.05, Friedman’s test for four related samples). Similar trends were observed in the level of uIL-18 or uCysC in non-AKI neonates that have at least three urinary samples during the first 10 days of life (n = 37, p > 0.05, Friedman’s test for three related samples).
Determination of how the concentration of uIL-18 and uCysC varies with gestational age and other factors in non-AKI control neonates
Univariate regression analysis demonstrated that neither the first uIL-18 nor the peak uIL-18 was significantly associated with gestational age, birth weight, body weight, postnatal age, gender, 5-min Apgar score, and SNAP in neonates without AKI. In contrast, both the first and the peak uCysC were significantly negatively associated with gestational age, birth weight, and body weight, but not with postnatal age, gender, 5-min Apgar score, and SNAP. The correlation analysis of the peak value of urinary biomarkers with gestational age, body weight, and postnatal age at sampling in non-AKI control neonates is shown in Fig. 1.
Correlation analysis of data from non-AKI (acute kidney injury) control neonates (n = 51). There was no significant correlation between the peak urinary interleukin-18 level and gestational age (a R2 = 0.005, p = 0.630), body weight (b R2 = 0.016, p = 0.386) or postnatal age at sampling (c R2 = 0.070, p = 0.062). The peak urinary cystatin C level was significantly negatively correlated with gestational age (d Slope = −342.6, intercept = 13397, R2 = 0.243, p = 0.000) and body weight (e slope = −1.394, intercept = 4575, R2 = 0.173, p = 0.003), but not with postnatal age at sampling (f R2 = 0.023, p = 0.291). Statistical analysis: univariate linear regression
In addition, using a stepwise multiple linear regression analysis, in which uIL-18 or uCysC was modeled with gestational and postnatal age, body weight, gender, Apgar score, and SNAP, the concentration of uCysC in non-AKI control neonates was only dependent on gestational age (the first uCysC: β = −0.520, p = 0.000; the peak uCysC: β = −0.549, p = 0.000).
Comparison of the peak value of uIL-18 and uCysC between AKI and non-AKI neonates
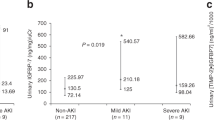
The peak concentration of both uIL-18 and uCysC was significantly higher in non-septic critically ill neonates with AKI as compared with non-AKI controls (Fig. 2a and b). The difference remained significant after controlling for gestational age, body weight, or gender using ANCOVA.
Association of urinary level of IL-18 and CysC with AKI
To identify whether uIL-18 and uCysC would predict the development of subsequent AKI in non-septic critically ill neonates, we analyzed the urine samples collected 0–48 h prior to the clinical diagnosis of AKI. The results of urinary biomarkers from the first collected urine samples of life were used to serve as controls in 51 neonates who did not develop AKI during the study period.
Logistic regression analysis identified that gestational age at birth, birth weight, 5-min Apgar score, SNAP, uIL-18, and uCysC were significantly associated with the development of AKI. The odds ratio for predicting the development of AKI is shown in Table 2. The association was further analyzed with adjustment for potential confounding variables. The 5-min Apgar score was not significantly associated with AKI after adjustment for gestational age at birth. The SNAP score, however, remained predictive of AKI development after adjustment for the same variable, and after further adjustment for postnatal age, birth weight, gender, and 5-min Apgar score. The level of uIL-18 and uCysC remained predictive of AKI development after adjustment for potential confounders, including gestational and postnatal age, birth weight, gender, 5-min Apgar score, and SNAP.
Ability of urinary level of IL-18 and CysC to predict AKI
The diagnostic performance of urinary biomarkers on predicting AKI development is assessed in Table 3. The level of uIL-18 was predictive of the development of AKI within 48 h (AUC = 0.72, p = 0.021). Urinary CysC was highly predictive of AKI, which displayed the best predictive performance, had odds ratio of 2.07, and achieved AUC of 0.92 for predicting the development of AKI within 48 h. In Fig. 3, we show that uCysC was better than uIL-18 or SNAP in predicting the development of AKI.
Receiver operating characteristic (ROC) curves for the ability of urinary biomarkers and the score for neonatal acute physiology (SNAP) to predict acute kidney injury (AKI) development in non-septic critically ill neonates (n = 62). The area under the ROC curve for urinary cystatin C (ng/mg uCr), urinary interleukin-18 (pg/mg uCr), and the score for neonatal acute physiology (SNAP) were 0.92, 0.72, and 0.73, respectively, with a Hosmer-Lemeshow goodness-of-fit p value >0.05
We also calculated the cut-off value for urinary biomarkers to predict the development of AKI within 48 h in Table 3. Urinary IL-18 displayed sensitivity of 64% and specificity of 92% at the optimal cut-off value of 1,800 pg/mg uCr. The positive and negative likelihood ratios were 8.0 and 0.4. At the concentration of uCysC ≥ 2,500 ng/mg uCr, the sensitivity and specificity for detecting AKI within 48 h were 91% and 86%, and the positive and negative likelihood ratios were 6.5 and 0.1, respectively.
Discussion
Our data demonstrate that AKI is characterized by high levels of uIL-18 and uCysC, which fits our hypothesis. Both uIL-18 and uCysC are predictive of subsequent development of AKI in non-septic critically ill neonates, independent of potential confounders.
The present study examines the impact of gestational and postnatal age, birth weight, gender, Apgar score, and SNAP on the utility of uIL-18 and uCysC as an AKI biomarker. Controlling for these important confounders is essential in interpreting AKI biomarkers in the context of the clinical status of the newborns.
Compared to the previous study in premature infants, our study included a neonatal population with a wide range of gestational age. Nevertheless, our results are in line with the previous study [26]. The level of uCysC, but not of uIL-18, decreased with increasing gestational age and birth weight. Furthermore, the present study demonstrates that uCysC decreased with increasing body weight, but not with increasing postnatal age in non-AKI control neonates during early life. It is well known that there is a significant body water loss during the first week of life in neonates. The decreased uCysC level in neonates with increasing gestational age and body weight might reflect renal maturity. The decreased excretion of urinary proteins with increasing renal maturation has been well elucidated in previous studies [34–36]. It may not be surprising that uIL-18 is not associated with gestational age, body weight, and postnatal age, because uIL-18 is released in response to renal tubular injury, and is not a marker of clearance [18].
In addition, there was a significant difference between AKI and non-AKI neonates with regard to gestational age, birth weight, Apgar score, respiratory distress syndrome (RDS), and mechanical ventilation in this study, which is consistent with previous studies. Low birth weight, low Apgar score and RDS were identified as independent risk factors for impaired renal function in neonates [7, 37].
To our knowledge, the present study is the first to examine the relationship between uIL-18 or uCysC and AKI development in critically ill neonates without sepsis during the first week of life. Neonates with systemic infections, including sepsis, were excluded in the present study, since it has been suggested that sepsis is significantly associated with the level of both uIL-18 and uCysC [14, 19]. It is important to assess potential biomarkers in a heterogeneous population of critically ill neonates with a wide range of gestational ages. In this real-world clinical setting, the kidney undergoes growth and maturation, and many risk factors are correlated with AKI and the timing of kidney injury is unknown. Focusing on a very early period after birth is also relevant, since a neonate during the first week of life is in a different physiological state due to the abrupt change at birth [36].
Urinary IL-18 is useful for the detection of AKI in non-septic critically ill neonates in our study, which is in agreement with a previous report from Washburn et al., where urinary IL-18 predicts severity of AKI only in non-septic critically ill children [21]. The most likely reason for the discrepancy between our data and the data from Askenazi et al. [27] is that the neonates with systemic infections, including sepsis, were excluded in the present study. In addition, the level of uIL-18 is not influenced by gestational and postnatal age, body weight, gender, Apgar score, and the severity of illness assessed by SNAP, which might be considered as advantages in the clinical utility of uIL-18 as an AKI biomarker in the neonatal population.
Urinary CysC is also associated with AKI development in the study, which is in line with the previous report [27]. One of our major findings was that uCysC displayed better performance, predicting the development of AKI with greater accuracy in non-septic critically ill neonates. One possible explanation might be that AKI definition is based on serum Cr, not renal tubular injury. Unlike uIL-18, the level of uCysC normalized to urinary Cr concentration has been shown to be a reliable screening tool for detecting decreased GFR in children [38]. Although CysC is most predominately reabsorbed from the proximal tubular cells, a detectable amount is still excreted in urine. The concentration of uCysC is found to reflect GFR when the ratio of urinary CysC to urinary Cr is in the normal range [39].
In addition, the performance of SNAP, which was based on 28 items collected over the first 24 h of admission to NICU in our study [28], merits further investigation. SNAP is suggested to be a measure of illness severity and correlates well with neonatal mortality [40]. Our study suggests that SNAP has the potential to be used in clinical settings as a method of predicting AKI development during the first week of life in critically ill neonates.
There were a number of limitations to our study. First, the definition of AKI in our study was based on the increase in serum Cr, which remains an accepted and widely used method for evaluating renal function in NICU. Since there is the lack of a classification system to define AKI in neonatal population, the difference in the definition of AKI might be likely to cause discrepancies when comparing results across studies. Second, we used the first urine samples prior to the clinical diagnosis of AKI collected from AKI neonates and the first urine samples of life collected from neonates who did not develop AKI during the first 10 days of life, which causes discrepancy in postnatal age at sampling. Notably, the level of neither uIL-18 nor uCysC was significantly affected by postnatal age. Third, we note that we did not follow daily urinary biomarkers and serum Cr as part of the study. The exact timing of kidney injury is uncertain. This may have led us to underestimate the rate of AKI and limited our ability to determine the exact time at which the levels of uIL-18 and uCysC rise prior to serum Cr increase, and to further evaluate the diagnostic performance at the time points. Fourth, there was a significantly higher mortality rate in neonates with AKI as compared with those without AKI in this study. However, the relatively small sample size limited our ability to determine whether urinary biomarkers can predict neonatal mortality.
In conclusion, our study indicates that the level of uCysC, but not uIL-18, may decrease with increasing renal maturity of the neonates. Both uIL-18 and uCysC may have an important role in non-septic critically ill neonates, as a non-invasive biomarker to independently predict AKI development during early life after birth. Urinary CysC could, as an independent biomarker with greater accuracy than uIL-18, predict the development of AKI in non-septic critically ill neonates. Large studies are needed to further explore the role of urinary biomarkers for detection of AKI in the neonatal population.
References
Askenazi DJ, Griffin R, McGwin G, Carlo W, Ambalavanan N (2009) Acute kidney injury is independently associated with mortality in very low birthweight infants: a matched case–control analysis. Pediatr Nephrol 24:991–997
Toth-Heyn P, Drukker A, Guignard JP (2000) The stressed neonatal kidney: from pathophysiology to clinical management of neonatal vasomotor nephropathy. Pediatr Nephrol 14:227–239
Askenazi DJ, Ambalavanan N, Goldstein SL (2009) Acute kidney injury in critically ill newborns: what do we know? What do we need to learn? Pediatr Nephrol 24:265–274
Andreoli SP (2004) Acute renal failure in the newborn. Semin Perinatol 28:112–123
Drukker A, Guignard JP (2002) Renal aspects of the term and preterm infant: a selective update. Curr Opin Pediatr 14:175–182
Gallini F, Maggio L, Romagnoli C, Marrocco G, Tortorolo G (2000) Progression of renal function in preterm neonates with gestational age < or =32 weeks. Pediatr Nephrol 15:119–124
Cuzzolin L, Fanos V, Pinna B, di Marzio M, Perin M, Tramontozzi P, Tonetto P, Cataldi L (2006) Postnatal renal function in preterm newborns: a role of diseases, drugs and therapeutic interventions. Pediatr Nephrol 21:931–938
Parikh CR, Lu JC, Coca SG, Devarajan P (2010) Tubular proteinuria in acute kidney injury: a critical evaluation of current status and future promise. Ann Clin Biochem 47:301–312
Devarajan P (2008) The future of pediatric acute kidney injury management–biomarkers. Semin Nephrol 28:493–498
Bagshaw SM, Bellomo R (2007) Early diagnosis of acute kidney injury. Curr Opin Crit Care 13:638–644
Doi K, Negishi K, Ishizu T, Katagiri D, Fujita T, Matsubara T, Yahagi N, Sugaya T, Noiri E (2011) Evaluation of new acute kidney injury biomarkers in a mixed intensive care unit. Crit Care Med 39:2464–2469
Parikh CR, Abraham E, Ancukiewicz M, Edelstein CL (2005) Urine IL-18 is an early diagnostic marker for acute kidney injury and predicts mortality in the intensive care unit. J Am Soc Nephrol 16:3046–3052
Parikh CR, Jani A, Melnikov VY, Faubel S, Edelstein CL (2004) Urinary interleukin-18 is a marker of human acute tubular necrosis. Am J Kidney Dis 43:405–414
Nejat M, Pickering JW, Walker RJ, Westhuyzen J, Shaw GM, Frampton CM, Endre ZH (2010) Urinary cystatin C is diagnostic of acute kidney injury and sepsis, and predicts mortality in the intensive care unit. Crit Care 14:R85
Koyner JL, Bennett MR, Worcester EM, Ma Q, Raman J, Jeevanandam V, Kasza KE, O'Connor MF, Konczal DJ, Trevino S, Devarajan P, Murray PT (2008) Urinary cystatin C as an early biomarker of acute kidney injury following adult cardiothoracic surgery. Kidney Int 74:1059–1069
Royakkers AA, Korevaar JC, van Suijlen JD, Hofstra LS, Kuiper MA, Spronk PE, Schultz MJ, Bouman CS (2011) Serum and urine cystatin C are poor biomarkers for acute kidney injury and renal replacement therapy. Intensive Care Med 37:493–501
Haase M, Bellomo R, Story D, Davenport P, Haase-Fielitz A (2008) Urinary interleukin-18 does not predict acute kidney injury after adult cardiac surgery: a prospective observational cohort study. Crit Care 12:R96
Melnikov VY, Faubel S, Siegmund B, Lucia MS, Ljubanovic D, Edelstein CL (2002) Neutrophil-independent mechanisms of caspase-1- and IL-18-mediated ischemic acute tubular necrosis in mice. J Clin Investig 110:1083–1091
Tschoeke SK, Oberholzer A, Moldawer LL (2006) Interleukin-18: a novel prognostic cytokine in bacteria-induced sepsis. Crit Care Med 34:1225–1233
Malyszko J (2010) Biomarkers of acute kidney injury in different clinical settings: a time to change the paradigm? Kidney Blood Press Res 33:368–382
Washburn KK, Zappitelli M, Arikan AA, Loftis L, Yalavarthy R, Parikh CR, Edelstein CL, Goldstein SL (2008) Urinary interleukin-18 is an acute kidney injury biomarker in critically ill children. Nephrol Dial Transplant 23:566–572
Finney H, Newman DJ, Thakkar H, Fell JM, Price CP (2000) Reference ranges for plasma cystatin C and creatinine measurements in premature infants, neonates, and older children. Arch Dis Child 82:71–75
Villa P, Jimenez M, Soriano MC, Manzanares J, Casasnovas P (2005) Serum cystatin C concentration as a marker of acute renal dysfunction in critically ill patients. Crit Care 9:R139–R143
Conti M, Moutereau S, Zater M, Lallali K, Durrbach A, Manivet P, Eschwege P, Loric S (2006) Urinary cystatin C as a specific marker of tubular dysfunction. Clin Chem Lab Med 44:288–291
Herget-Rosenthal S, van Wijk JA, Brocker-Preuss M, Bokenkamp A (2007) Increased urinary cystatin C reflects structural and functional renal tubular impairment independent of glomerular filtration rate. Clin Biochem 40:946–951
Askenazi DJ, Koralkar R, Levitan EB, Goldstein SL, Devarajan P, Khandrika S, Mehta RL, Ambalavanan N (2011) Baseline values of candidate urine acute kidney injury biomarkers vary by gestational age in premature infants. Pediatr Res 70:302–306
Askenazi DJ, Montesanti A, Hunley H, Koralkar R, Pawar P, Shuaib F, Liwo A, Devarajan P, Ambalavanan N (2011) Urine biomarkers predict acute kidney injury and mortality in very low birth weight infants. J Pediatr 159:907–912
Dorling JS, Field DJ, Manktelow B (2005) Neonatal disease severity scoring systems. Arch Dis Child Fetal Neonatal Ed 90:F11–F16
Brion LP, Fleischman AR, McCarton C, Schwartz GJ (1986) A simple estimate of glomerular filtration rate in low birth weight infants during the first year of life: noninvasive assessment of body composition and growth. J Pediatr 109:698–707
Schwartz GJ, Feld LG, Langford DJ (1984) A simple estimate of glomerular filtration rate in full-term infants during the first year of life. J Pediatr 104:849–854
Akcan-Arikan A, Zappitelli M, Loftis LL, Washburn KK, Jefferson LS, Goldstein SL (2007) Modified RIFLE criteria in critically ill children with acute kidney injury. Kidney Int 71:1028–1035
Zappitelli M, Washburn KK, Arikan AA, Loftis L, Ma Q, Devarajan P, Parikh CR, Goldstein SL (2007) Urine neutrophil gelatinase-associated lipocalin is an early marker of acute kidney injury in critically ill children: a prospective cohort study. Crit Care 11:R84
Melnikov VY, Ecder T, Fantuzzi G, Siegmund B, Lucia MS, Dinarello CA, Schrier RW, Edelstein CL (2001) Impaired IL-18 processing protects caspase-1-deficient mice from ischemic acute renal failure. J Clin Investig 107:1145–1152
Fell JM, Thakkar H, Newman DJ, Price CP (1997) Measurement of albumin and low molecular weight proteins in the urine of newborn infants using a cotton wool ball collection method. Acta Paediatr 86:518–522
Tsukahara H, Hiraoka M, Kuriyama M, Saito M, Morikawa K, Kuroda M, Tominaga T, Sudo M (1993) Urinary alpha 1-microglobulin as an index of proximal tubular function in early infancy. Pediatr Nephrol 7:199–201
Zelenina M, Li Y, Glorieux I, Arnaud C, Cristini C, Decramer S, Aperia A, Casper C (2006) Urinary aquaporin-2 excretion during early human development. Pediatr Nephrol 21:947–952
Tulassay T, Vasarhelyi B (2002) Birth weight and renal function. Curr Opin Nephrol Hypertens 11:347–352
Hellerstein S, Berenbom M, Erwin P, Wilson N, DiMaggio S (2004) The ratio of urinary cystatin C to urinary creatinine for detecting decreased GFR. Pediatr Nephrol 19:521–525
Uchida K, Gotoh A (2002) Measurement of cystatin-C and creatinine in urine. Clin Chim Acta 323:121–128
Kumar D, Super DM, Fajardo RA, Stork EE, Moore JJ, Saker FA (2004) Predicting outcome in neonatal hypoxic respiratory failure with the score for neonatal acute physiology (SNAP) and highest oxygen index (OI) in the first 24 hours of admission. J Perinatol 24:376–381
Acknowledgements
We thank the staff in biochemistry laboratory for technical assistance. This work was supported by grants from the National Natural Science Foundation of China (30972711) and the National Natural Science Foundation of Jiangsu (BK2009128).
Author information
Authors and Affiliations
Corresponding author
Additional information
Yanhong Li, Xiaozhong Li, and Xing Feng contributed equally to this work.
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License ( https://creativecommons.org/licenses/by-nc/2.0 ), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Li, Y., Fu, C., Zhou, X. et al. Urine interleukin-18 and cystatin-C as biomarkers of acute kidney injury in critically ill neonates. Pediatr Nephrol 27, 851–860 (2012). https://doi.org/10.1007/s00467-011-2072-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-011-2072-x