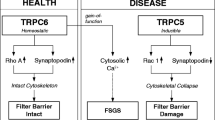
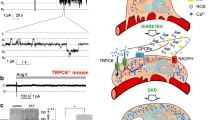
Abstract
Glomerular kidney disease is a major healthcare burden and considered to represent a sum of disorders that evade a refined and effective treatment. Excellent biological and genetic studies have defined pathways that go awry in podocytes, which are the regulatory cells of the glomerular filter. The question now is how to define targets for novel improved therapies. In this review, we summarize critical points around targeting the TRPC6 channel in podocytes.



Similar content being viewed by others
References
Hogg RJ, Portman RJ, Milliner D, Lemley KV, Eddy A, Ingelfinger J (2000) Evaluation and management of proteinuria and nephrotic syndrome in children: recommendations from a pediatric nephrology panel established at the National Kidney Foundation conference on proteinuria, albuminuria, risk, assessment, detection, and elimination (PARADE). Pediatrics 105(6):1242–1249
Reiser J, Gupta V, Kistler AD (2010) Toward the development of podocyte-specific drugs. Kidney Int 77(8):662–628
Möller CC, Flesche J, Reiser J (2009) Sensitizing the slit diaphragm with TRPC6 ion channels. J Am Soc Nephrol 20(5):950–953
Reiser J, Polu KR, Möller CC, Kenlan P, Altintas MM, Wei C, Faul C, Herbert S, Villegas I, Avila-Casado C, McGee M, Sugimoto H, Brown D, Kalluri R, Mundel P, Smith PL, Clapham DE, Pollak MR (2005) TRPC6 is a glomerular slit diaphragm-associated channel required for normal renal function. Nat Genet 37(7):739–744
Faul C, Asanuma K, Yanagida-Asanuma E, Kim K, Mundel P (2007) Actin up: regulation of podocyte structure and function by components of the actin cytoskeleton. Trends Cell Biol 17(9):428–437
Rodewald R, Karnovsky MJ (1974) Porous substructure of the glomerular slit diaphragm in the rat and mouse. J Cell Biol 60(2):423–433
Reiser J, Kriz W, Kretzler M, Mundel P (2000) The glomerular slit diaphragm is a modified adherens junction. J Am Soc Nephrol 11(1):1–8
Wartiovaara J, Ofverstedt LG, Khoshnoodi J, Zhang J, Mäkelä E, Sandin S, Ruotsalainen V, Cheng RH, Jalanko H, Skoglund U, Tryggvason K (2004) Nephrin strands contribute to a porous slit diaphragm scaffold as revealed by electron tomography. J Clin Invest 14(10):1475–1483
Tryggvason K, Wartiovaara J (2001) Molecular basis of glomerular permselectivity. Curr Opin Nephrol Hypertens 10(4):543–549
Ruotsalainen V, Ljungberg P, Wartiovaara J, Lenkkeri U, Kestilä M, Jalanko H, Holmberg C, Tryggvason K (1999) Nephrin is specifically located at the slit diaphragm of glomerular podocytes. Proc Natl Acad Sci USA 96(14):7962–7967
Rantanen M, Palmén T, Pätäri A, Ahola H, Lehtonen S, Aström E, Floss T, Vauti F, Wurst W, Ruiz P, Kerjaschki D, Holthöfer H (2002) Nephrin TRAP mice lack slit diaphragms and show fibrotic glomeruli and cystic tubular lesions. J Am Soc Nephrol 13(6):1586–1594
Chuang PY, He JC (2009) Signaling in regulation of podocyte phenotypes. Nephron Physiol 111(2):9–15
Clapham DE (2003) TRP channels as cellular sensors. Nature 426(6966):517–524
Venkatachalam K, Montell C (2007) TRP channels. Annu Rev Biochem 76:387–417
Eid SR, Cortright DN (2009) Transient receptor potential channels on sensory nerves. Handb Exp Pharmacol 194:261–281
Kiselyov K, Xu X, Kuo TH, Mozhayeva G, Pessah I, Mignery G, Zhu X, Birnbaumer L, Muallem S (1998) Functional interaction between InsP3 receptors and store-operated Htrp3 channels. Nature 396(6710):478–482
Kiselyov K, Mignery GA, Zhu MX, Muallem S (1999) The N-terminal domain of the IP3 receptor gates store-operated hTrp3 channels. Mol Cell 4(3):423–429
Zhu MX (2005) Multiple roles of calmodulin and other Ca(2+)-binding proteins in the functional regulation of TRP channels. Pflugers Arch 451(1):105–115
Hofmann T, Schaefer M, Schultz G, Gudermann T (2002) Subunit composition of mammalian transient receptor potential channels in living cells. Proc Natl Acad Sci USA 99(11):7461–7466
Dryer SE, Reiser J (2010) TRPC6 channels and their binding partners in podocytes: role in glomerular filtration and pathophysiology. Am J Physiol Renal Physiol 299(4):F689–F701
Winn MP, Conlon PJ, Lynn KL, Farrington MK, Creazzo T, Hawkins AF, Daskalakis N, Kwan SY, Ebersviller S, Burchette JL, Pericak-Vance MA, Howell DN, Vance JM, Rosenberg PB (2005) A mutation in the TRPC6 cation channel causes familial focal segmental glomerulosclerosis. Science 308(5729):1801–1804
Kestilä M, Lenkkeri U, Männikkö M, Lamerdin J, McCready P, Putaala H, Ruotsalainen V, Morita T, Nissinen M, Herva R, Kashtan CE, Peltonen L, Holmberg C, Olsen A, Tryggvason K (1998) Positionally cloned gene for a novel glomerular protein–nephrin–is mutated in congenital nephrotic syndrome. Mol Cell 11(4):575–582
Boute N, Gribouval O, Roselli S, Benessy F, Lee H, Fuchshuber A, Dahan K, Gubler MC, Niaudet P, Antignac C (2000) NPHS2, encoding the glomerular protein podocin, is mutated in autosomal recessive steroid-resistant nephrotic syndrome. Nat Genet 24(4):349–354
Niaudet P, Gubler MC (2006) WT1 and glomerular diseases. Pediatr Nephrol 21(11):1653–1660
Kaplan JM, Kim SH, North KN, Rennke H, Correia LA, Tong HQ, Mathis BJ, Rodríguez-Pérez JC, Allen PG, Beggs AH, Pollak MR (2000) Mutations in ACTN4, encoding alpha-actinin-4, cause familial focal segmental glomerulosclerosis. Nat Genet 24(3):251–256
Schaefer M (2005) Homo- and heteromeric assembly of TRP channel subunits. Pflugers Arch 451(1):35–42
Dietrich A, Chubanov V, Kalwa H, Rost BR, Gudermann T (2006) Cation channels of the transient receptor potential superfamily: their role in physiological and pathophysiological processes of smooth muscle cells. Pharmacol Ther 112(3):744–760
Shih NY, Li J, Karpitskii V, Nguyen A, Dustin ML, Kanagawa O, Miner JH, Shaw AS (1999) Congenital nephrotic syndrome in mice lacking CD2-associated protein. Science 286(5438):312–315
Grunkemeyer JA, Kwoh C, Huber TB, Shaw AS (2005) CD2-associated protein (CD2AP) expression in podocytes rescues lethality of CD2AP deficiency. J Biol Chem 280(33):29677–29681
Schindl R, Romanin C (2007) Assembly domains in TRP channels. Biochem Soc Trans 5(Pt 1):84–85
Estacion M, Li S, Sinkins WG, Gosling M, Bahra P, Poll C, Westwick J, Schilling WP (2004) Activation of human TRPC6 channels by receptor stimulation. J Biol Chem 279(21):22047–22056
Gudermann T, Hofmann T, Mederos y Schnitzler M, Dietrich A (2004) Activation, subunit composition and physiological relevance of DAG-sensitive TRPC proteins. Novartis Found Symp 258:103–118, discussion 118–122, 155–159, 263–266
Wedel BJ, Vazquez G, McKay RR, Bird GStJ, Putney JW Jr (2003) A calmodulin/inositol 1,4,5-trisphosphate (IP3) receptor-binding region targets TRPC3 to the plasma membrane in a calmodulin/IP3 receptor-independent process. J Biol Chem 278(28):25758–25765
Smyth JT, Lemonnier L, Vazquez G, Bird GS, Putney JW Jr (2006) Dissociation of regulated trafficking of TRPC3 channels to the plasma membrane from their activation by phospholipase C. J Biol Chem 281(17):11712–11720
Cayouette S, Lussier MP, Mathieu EL, Bousquet SM, Boulay G (2004) Exocytotic insertion of TRPC6 channel into the plasma membrane upon Gq protein-coupled receptor activation. J Biol Chem 279(8):7241–7246
Heeringa SF, Möller CC, Du J, Yue L, Hinkes B, Chernin G, Vlangos CN, Hoyer PF, Reiser J, Hildebrandt F (2009) A novel TRPC6 mutation that causes childhood FSGS. PLoS One 4(11):e7771
Lepage PK, Lussier MP, Barajas-Martinez H, Bousquet SM, Blanchard AP, Francoeur N, Dumaine R, Boulay G (2006) Identification of two domains involved in the assembly of transient receptor potential canonical channels. J Biol Chem 281(41):30356–30364
Hinkes BG, Mucha B, Vlangos CN, Gbadegesin R, Liu J, Hasselbacher K, Hangan D, Ozaltin F, Zenker M, Hildebrandt F, Arbeitsgemeinschaft für Paediatrische Nephrologie Study Group (2007) Nephrotic syndrome in the first year of life: two thirds of cases are caused by mutations in 4 genes (NPHS1, NPHS2, WT1, and LAMB2). Pediatrics 119(4):e907–e919
Möller CC, Wei C, Altintas MM, Li J, Greka A, Ohse T, Pippin JW, Rastaldi MP, Wawersik S, Schiavi S, Henger A, Kretzler M, Shankland SJ, Reiser J (2007) Induction of TRPC6 channel in acquired forms of proteinuric kidney disease. J Am Soc Nephrol 18(1):29–36
Ronco P, Debiec H (2006) New insights into the pathogenesis of membranous glomerulonephritis. Curr Opin Nephrol Hypertens 15(3):258–263
Glassock RJ (2004) The treatment of idiopathic membranous nephropathy: a dilemma or a conundrum? Am J Kidney Dis 44(3):562–566
Beck LH Jr, Bonegio RG, Lambeau G, Beck DM, Powell DW, Cummins TD, Klein JB, Salant DJ (2009) M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med 361(1):11–21
Imai H, Hamai K, Komatsuda A, Ohtani H, Miura AB (1997) IgG subclasses in patients with membranoproliferative glomerulonephritis, membranous nephropathy, and lupus nephritis. Kidney Int 51(1):270–276
Pippin JW, Durvasula R, Petermann A, Hiromura K, Couser WG, Shankland SJ (2003) DNA damage is a novel response to sublytic complement C5b-9-induced injury in podocytes. J Clin Invest 111(6):877–885
Cybulsky AV, Bonventre JV, Quigg RJ, Lieberthal W, Salant DJ (1990) Cytosolic calcium and protein kinase C reduce complement-mediated glomerular epithelial injury. Kidney Int 38(5):803–811
Topham PS, Haydar SA, Kuphal R, Lightfoot JD, Salant DJ (1999) Complement-mediated injury reversibly disrupts glomerular epithelial cell actin microfilaments and focal adhesions. Kidney Int 55(5):1763–1775
Brooks RC, McCarthy KD, Lapetina EG, Morell P (1989) Receptor-stimulated phospholipase A2 activation is coupled to influx of external calcium and not to mobilization of intracellular calcium in C62B glioma cells. J Biol Chem 264(33):20147–20153
Wenzel RR (2005) Renal protection in hypertensive patients: selection of antihypertensive therapy. Drugs 65[Suppl 2]:29–39
Alaniz C, Brosius FC 3rd, Palmieri J (1993) Pharmacologic management of adult idiopathic nephrotic syndrome. Clin Pharm 12(6):429–439
Hauser PV, Pippin JW, Kaiser C, Krofft RD, Brinkkoetter PT, Hudkins KL, Kerjaschki D, Reiser J, Alpers CE, Shankland SJ (2010) Novel siRNA delivery system to target podocytes in vivo. PLoS One 5(3):e9463
Chiang WC, Geel TM, Altintas MM, Sever S, Ruiters MH, Reiser J (2010) Establishment of protein delivery systems targeting podocytes. PLoS One 5(7):e11837
Hartleben B, Gödel M, Meyer-Schwesinger C, Liu S, Ulrich T, Köbler S, Wiech T, Grahammer F, Arnold SJ, Lindenmeyer MT, Cohen CD, Pavenstädt H, Kerjaschki D, Mizushima N, Shaw AS, Walz G, Huber TB (2010) Autophagy influences glomerular disease susceptibility and maintains podocyte homeostasis in aging mice. J Clin Invest 120(4):1084–1096
Schlöndorff J, Del Camino D, Carrasquillo R, Lacey V, Pollak MR (2009) TRPC6 mutations associated with focal segmental glomerulosclerosis cause constitutive activation of NFAT-dependent transcription. Am J Physiol Cell Physiol 296(3):C558–C569
Acknowledgments
This work was supported in part by US National Institutes of Health (NIH) grants DK073495 and DK089394 to J.R. The authors thank Jim Stanis for help with the illustration of Fig. 3.
Author information
Authors and Affiliations
Corresponding author
Additional information
Answers
1) c
2) a
3) d
4) a
5) d
Questions
Questions
Answers appear following the reference list.
-
1.
Which is the key cell in the kidney that regulates filtration?
-
a)
mesangial cell
-
b)
proximal tubular epithelial cell
-
c)
podocyte
-
d)
parietal epithelial cell
-
e)
endothelial cell
-
a)
-
2.
The podocyte is most similar to ?
-
a)
pericyte
-
b)
vascular smooth muscle cell
-
c)
adipocyte
-
d)
fibroblast
-
e)
none of the above
-
a)
-
3.
The glomerular slit diaphragm is ?
-
a)
a microvillus
-
b)
an intracellular contact
-
c)
an extension of the GBM
-
d)
a modified adherens junction
-
e)
a classical tight junction
-
a)
-
4.
TRPC6 is potentially a good drug target because ?
-
a)
it is involved in regulation of the kidney filter
-
b)
it is the main gene mutated in IgA nephropathy
-
c)
it is the TRP channel in polycystic kidney disease
-
e)
none of the above
-
a)
-
5.
Podocytes are druggable cells because ?
-
a)
they turn over frequently
-
b)
they are exposed to blood and primary urine
-
c)
they display active endo- and macropinocytosis
-
d)
b + c are correct
-
e)
none of the above
-
a)
Rights and permissions
About this article
Cite this article
El Hindi, S., Reiser, J. TRPC channel modulation in podocytes—inching toward novel treatments for glomerular disease. Pediatr Nephrol 26, 1057–1064 (2011). https://doi.org/10.1007/s00467-010-1718-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-010-1718-4