Abstract
Background
The surgical resection of rectal carcinoma is associated with a high risk of permanent stoma rate. Primary anastomosis rate is suggested to be higher in robot-assisted and transanal total mesorectal excision, but permanent stoma rate is unknown.
Methods
Patients undergoing total mesorectal excision for MRI-defined rectal cancer between 2015 and 2017 in 11 centers highly experienced in laparoscopic, robot-assisted or transanal total mesorectal excision were included in this retrospective study. Permanent stoma rate, stoma-related complications, readmissions, and reoperations were registered. A multivariable regression analysis was performed for permanent stoma rate, stoma-related complications, and stoma-related reoperations.
Results
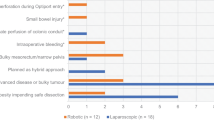
In total, 1198 patients were included. Permanent stoma rate after low anterior resection (with anastomosis or with an end colostomy) was 40.1% in patients undergoing laparoscopic surgery, 21.3% in patients undergoing robot-assisted surgery, and 25.6% in patients undergoing transanal surgery (P < 0.001). Permanent stoma rate after low anterior resection with an anastomosis was 17.3%, 11.8%, and 15.1%, respectively. The robot-assisted and transanal techniques were independently associated with a reduction in permanent stoma rate in patients who underwent a low anterior resection (with anastomosis or with an end colostomy) (OR 0.39 [95% CI 0.25, 0.59] and OR 0.35 [95% CI 0.22, 0.55]), while this was not seen in patients who underwent a restorative low anterior resection. 45.4% of the patients who had a stoma experienced stoma-related complications, 4.0% were at least once readmitted, and 8.9% underwent at least one reoperation.
Conclusions
The robot-assisted and transanal techniques are associated with a lower permanent stoma rate in patients who underwent a low anterior resection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Total mesorectal excision (TME) is the standard treatment for rectal cancer. It can be performed using open, laparoscopic (L-TME), robot-assisted (R-TME), and transanal TME (TaTME) [1], and is associated with a high incidence of stoma construction [2, 3].
Based on tumor characteristics and patient preferences, a low anterior resection (LAR) with or without the construction of an anastomosis, or an abdominoperineal resection (APR) may be performed. In patients undergoing an APR, and patients undergoing a LAR without anastomosis, an end colostomy is constructed. In patients undergoing a LAR with anastomosis, a temporary stoma may be considered, although the (dis)advantages are strongly debated [4,5,6]. While most diverting stomas and some end colostomies constructed during a LAR are intended to be reversed, up to 20% will never be reversed, and a considerable proportion of rectal cancer patients end up having a permanent stoma [7, 8].
Although it is unclear whether having a stoma influences quality of life, stoma-related complications are often described in patients having a stoma [9, 10]. As stoma-related complications are known to occur more frequently with an increased duration of the stoma, permanent stomas are suggested to have a high rate of stoma-related morbidity and might therefore result in a stronger decline in quality of life compared to patients having a temporary stoma [9,10,11,12,13]. Recent studies showed that R-TME and TaTME performed by experienced surgeons resulted in significantly more primary anastomoses, compared to L-TME [14,15,16,17]. These results suggest that R-TME and TaTME are better capable of constructing an anastomosis; however, it is unknown whether this effect will remain on the long term, and will result in a lower permanent stoma rate. Therefore, this study aims to compare L-TME, R-TME, and TaTME with regard to permanent stoma rate.
Materials and methods
This is a retrospective cohort study of rectal cancer patients operated between January 1st, 2015 and December 31st, 2017 in eleven large Dutch teaching centers, performed by experienced rectal cancer surgeons. As it is difficult to reach the same levels of proficiency in all three techniques by the same surgeon, we compared the results of high-volume hospitals specialized predominantly in one of the three techniques. Centers were ‘dedicated’ in either L-TME, R-TME, or TaTME and only one of the techniques was the standard technique.
Objectives
The primary objective was to determine the permanent stoma rate among all patients who underwent a LAR (with anastomosis or with an end colostomy). Secondary objectives included the permanent stoma rate among all L-TME, R-TME, and TaTME patients (this included LAR with colostomy, LAR with anastomosis and APR) and among all patients who underwent a restorative LAR (with the construction of an anastomosis). Other objectives included the determination of the incidence of stoma-related complications and reinterventions during long-term follow-up in all patients having a stoma.
Patients
Patients were eligible for inclusion if they (1) underwent a TME because of primary rectal cancer according to the rectal cancer definition as proposed by d’Souza et al. [18], (2) were operated between January 1st, 2015 and December 31st, 2017, (3) were registered in the national prospective Dutch Colo-Rectal Audit (DCRA) database, and (4) underwent minimally invasive TME. Patients were excluded if (1) they already had a stoma unrelated to treatment of the rectal cancer at diagnosis or (2) when they underwent a TME with palliative intent or due to recurrent disease.
Data
An already existing retrospective database aimed at comparing L-TME, R-TME, and TaTME was used [14]. In short, the database consisted of prospectively registered data from the DCRA, while missing data, and additional data not present in the DCRA database were added through the patients’ electronic medical record. Informed consent was waived by the regional medial ethical committee, and both the regional medical ethical committee and all local hospital ethical committees gave their approval for the study (MEC-U: AW19-023). The study design and drafting of the article were in accordance with the STROBE statement [19].
Outcomes
A permanent stoma was defined as a stoma created during the initial surgery or later, that remained until the patient’s last follow-up visit or death of the patient. A primary stoma was defined as a stoma constructed prior or during the TME, a secondary stoma was defined as a stoma constructed after the TME, and a tertiary stoma was defined as a stoma constructed after initial stoma reversal of the primary or secondary stoma. Stoma-related complications included ileus due to the stoma, high-output stoma, stoma prolapse, parastomal hernia, stricture of the stoma, dehiscence of the stoma, necrosis of the stoma, parastomal skin complications, and infectious complications. Stoma-related complications were defined as short term if they occurred within the first 30 days after stoma construction, and as long term if they occurred more than 30 days after stoma construction. Furthermore, the following data related to the stoma were registered: type of stoma (diverting ileostomy, end ileostomy, diverting colostomy, end colostomy), timing of stoma construction (prior to initial resection, during initial resection, after surgical resection as a consequence of surgical complications, after reversal), reversal of stoma, time to reversal, readmissions related to the stoma, and reoperations related to the stoma. Readmissions and reoperations related to reversal were not registered.
Baseline characteristics were age, body mass index (BMI), sex, American Society of Anesthesiologists’ (ASA) classification, history of abdominal surgery, use of neoadjuvant therapy, TNM stage [21], and distance from the inferior border of the tumor from the anorectal junction. Furthermore, type of minimally invasive TME-technique, type of surgical procedure, surgical complications, reinterventions, and anastomotic leakage were registered. Anastomotic leakage was defined according to the ISREC criteria [20], and registered during the whole follow-up period. Type of minimally invasive TME-technique was defined as L-TME, R-TME, and TaTME. In case of TaTME, the abdominal part of the procedure was performed laparoscopically. Type of the surgical procedure was defined as an APR, a LAR or a restorative LAR. An APR was defined as either an intersphincteric, a classic or an extralevatoric APR with proctectomy. A LAR could be either with the construction of an anastomosis or without the construction of an anastomosis but with an end ostomy. Finally, the group patients who underwent a restorative LAR all had an anastomosis constructed during the initial resection.
Statistical analysis
For the comparative analyses, outcomes of LAR (with anastomosis or with an end colostomy) were compared between the L-TME, R-TME, and TaTME technique. In addition, to account for selection bias regarding, outcomes were compared between all patients who underwent a L-TME, R-TME, or TaTME, thereby including the patients who underwent an APR. Furthermore, data were stratified per center (L-TME center, R-TME center and TaTME center) as well and added in the supplemental tables. The Fisher’s exact test was used for comparing categorical differences. The Wilcoxon-rank sum test was used for continuous variables that were not normally distributed data, whereas the student’s t test was used for normally distributed data.
To control for confounding factors, multivariable regression analyses were performed. Multivariable analyses were performed using backward logistic regression analysis for permanent stoma rate, stoma-related complications, and stoma-related reoperations. Variables included in the logistic regression analysis for permanent stoma rate of all TME patients (LAR with anastomosis or with an end colostomy, and APR) and for patients undergoing a LAR (with or without the construction of an anastomosis) were based on literature, and included age, gender, BMI, ASA classification, history of abdominal surgery, neoadjuvant therapy, cTNM stage, mesorectal fascia (MRF) involvement, tumor distance to the ARJ, type of minimally invasive TME-technique, and duration of follow-up. For the multivariable analysis of permanent stoma rate after restorative LAR (with the construction of an anastomosis), the following two variables were added as well: diverting stoma during primary resection and anastomotic leakage. For stoma complications and stoma interventions, the following risk factors were included: age, gender, BMI, ASA classification, history of abdominal surgery, neoadjuvant therapy, type of stoma, moment of stoma construction, and total duration of the stoma [22,23,24,25,26,27,28,29]. Missing data were imputed using multiple imputation if data were missing at random or if data were missing completely at random. Analyses were performed using’R’ version 4.1.3.
Results
Baseline characteristics
In total, 1834 patients were identified in the retrospective cohort; 1198 patients were included in the analysis. A TME was performed using the laparoscopic technique in 596 patients, the robot-assisted technique was used in 353 patients, whereas the transanal technique was performed in 249 patients. After excluding patients who underwent an APR, 344 patients remained in the laparoscopic group, 235 in the robot-assisted group, and 203 in the transanal group (Fig. 1). The majority of patients underwent surgery by the dedicated technique of the specific center. Patients in the TaTME centers were younger, had a lower tumor, and a shorter follow-up (Supplemental Table 1). In the TaTME centers, laparoscopic TME was used in 47 out of 90 patients who underwent an APR (48.0%), while laparoscopic TME was used in 15 out of 125 patients for APR in the R-TME centers (12.0%). Irrespective of the center, patients who underwent a TaTME were younger and had a lower tumor, and patients who underwent a TaTME or a R-TME less frequently had a history of abdominal surgery (Table 1).
Flow diagram of included patients. N: number of patients, L-TME: laparoscopic total mesorectal excision, R-TME: robot-assisted total mesorectal excision, TaTME: transanal total mesorectal excision, TEM: transanal endoscopic microsurgery, TAMIS: transanal minimally invasive surgery, APR: abdominoperineal resection
Stoma characteristics
In 4.9%, 8.5%, and 4.9% of the patients who underwent a LAR using the laparoscopic, robot-assisted, and transanal technique, respectively, a stoma was constructed prior to resection (P = 0.16). A significantly lower primary anastomosis rate was observed in patients who underwent a LAR using the laparoscopic technique compared to patients who underwent a LAR using the robot-assisted or transanal technique (72.4% versus 90.2% versus 88.2%, P < 0.001). After the initial resection, 70.9%, 72.3%, and 60.6% of the patients who underwent a LAR using the laparoscopic, robot-assisted, and transanal technique had a primary stoma, respectively (P < 0.001). Stoma construction due to a surgical complication, resulting in a secondary stoma, was observed in 7.3%, 5.1%, and 9.4% of the patients (P = 0.04). Construction of a new stoma after reversal (tertiary stoma) was performed in 4.9%, 6.4%, and 5.4% (P = 0.67) of the patients (Table 2).
Permanent stoma rate
A permanent stoma at the end of follow-up in patients who underwent a LAR was observed in 40.1%, 21.3%, and 25.6% of the patients in the laparoscopic, robot-assisted, and TaTME group, respectively (P < 0.001). For patients undergoing a restorative LAR, this was 17.3%, 11.8%, and 15.1% (P = 0.26), respectively (Tables 2 and 3). Reversal of a primary stoma in patients who underwent a restorative LAR was performed in 88.6%, 94.6%,and 87.9% of the laparoscopic, robot-assisted and transanal group (P = 0.19) (Table 3). Multivariable regression analysis of patients undergoing a LAR using the robot-assisted technique (OR 0.39 [95% CI 0.25, 0.59]) and transanal technique (OR 0.35 [95% CI 0.22, 0.55]) was associated with a lower permanent stoma rate compared to the laparoscopic technique. (Table 4) Other variables independently associated with permanent stoma rate were age, history of abdominal surgery, ASA classification, distance to the ARJ, and neoadjuvant therapy. In patients undergoing a restorative LAR, the multivariable regression analysis showed that R-TME and TaTME were not associated with permanent stoma rate. Variables independently associated with permanent stoma rate were history of abdominal surgery, ASA classification, distance to the ARJ, anastomotic leakage, and the construction of a diverting stoma during initial resection. (Table 4).
Stoma-related complications
Stoma complications were present in 39.1% of the patients with a diverting ileostomy, 66.7% of the patients with an end ileostomy, 44.1% of the patients with a diverting colostomy, and 49.6% of the patients with an end colostomy. Stoma complications within 30 days after the initial resection were mostly seen in patients with a diverting ileostomy. High-output stoma and ileus were most frequently seen in this group, while necrosis was most frequently seen in patients with an end colostomy. Stoma complications after 30 days were mostly seen in patients with an end colostomy, with parastomal hernia being the most frequent complication. Skin complications were seen in all four types of stomas. (Table 5) Multivariable regression analysis showed that years of having a stoma was independently associated with the occurrence of overall stoma complications (OR 1.29 [95% CI 1.19, 1.40]).
Stoma-related readmissions and reoperations
40 (4.0%) patients experienced one or more readmissions during the follow-up, with the majority of the patients (82.5%) being readmitted only once. Additionally, 89 patients (8.9%) underwent one or more reoperations. (Table 6) Multivariable regression analysis showed that a diverting colostomy (OR 4.83 [95% CI 1.88, 12.03]) and years of having a stoma (OR 1.83 [95% CI 1.52, 2.20]) were independently associated with stoma-related reoperations.
Discussion
This study aimed to evaluate permanent stoma rate in patients undergoing minimally invasive rectal cancer surgery. In this study, patients undergoing a LAR using the robot-assisted or transanal technique were independently associated with a lower permanent stoma rate compared to the laparoscopic technique. Additionally, a high rate of stoma-related complications, readmissions, and reoperations was observed.
Previous studies already suggested an increased anastomosis rate using the transanal and robot-assisted technique, without an increase in anastomotic leakage rate [14, 15, 17]. However, it was yet unclear whether this would also result in a lower permanent stoma rate. In this study, the robot-assisted and transanal techniques were independently associated with a lower permanent stoma rate in the total group of patients who underwent a TME, and in the group of patients who underwent a LAR. In patients who underwent a restorative LAR, these minimally invasive techniques were not associated with permanent stoma rate. This strengthens the hypothesis that the lower permanent stoma rate might be due to higher anastomosis rate using the robot-assisted and transanal techniques. Additionally, this suggests that the increased anastomotic rate using the robot-assisted and transanal techniques does not come with a higher anastomotic break-down during 3-year follow-up, resulting in a lower permanent stoma rate. The improved visibility in both techniques might explain the increase in primary anastomosis rate, and the subsequent associated lower permanent stoma rate. Perhaps, with enhanced visibility, stapling could be easier: the robot-assisted technique comes with more degrees of freedom, thereby potentially reducing stapling difficulties. The effect on permanent stoma rate was stronger in the transanal group (OR 0.17) than the robot-assisted group (OR 0.48) for all TME patients, while the effect was comparable between the transanal group (OR 0.35) and the robot-assisted group (OR 0.39) in patients who underwent a LAR. Probably this is because TaTME is mostly not used for an APR, while the laparoscopic approach is used in these cases. This causes selection bias, and therefore, the association of TaTME with permanent stoma rate will probably be lower. Nevertheless, after excluding patients with an APR, the association of the robot-assisted and transanal techniques with permanent stoma rate remains, supporting or hypothesis that the robot-assisted and transanal techniques are associated with a higher primary anastomosis rate and a lower permanent stoma rate.
Variables associated with permanent stoma rate other than the technique were age, history of abdominal surgery, ASA classification, lower distance to the ARJ, chemoradiation, anastomotic leakage, and diverting stoma during initial resection. Two recent large nation-wide studies showed comparable risk factors for permanent stoma rate. However, minimally invasive surgery was not associated with a reduced permanent stoma rate [30, 31], perhaps since they did not separately identify R-TME, L-TME, and TaTME. Or this is due to the fact that the current study only included patients operated in dedicated centers.
This study shows a significant rate of stoma-related complications: 45.4% of the patients experienced stoma-related complications, 4.0% of the patients were at least once readmitted, and 8.9% of the patients underwent at least one reoperation, irrespective of admission and reoperation related to reversal of a diverting stoma. This is in line with other studies showing considerable stoma-related complications, readmissions, and reoperations [32,33,34]. Despite the presented rates, stoma-related morbidity with a follow-up duration of only 36 months still might underestimate the actual morbidity, especially in permanent stomas, as the incidence of complications increases with the duration of having a stoma.
Although the robot-assisted and transanal techniques were associated with a lower permanent stoma rate, it is unclear how this will affect quality of life [35, 36]. It is suggested that quality of life might be better in patients with a restorative resection [9, 10]. However, some studies show worse quality of life in patients with restorative resections compared to patients with a permanent stomay [37, 38]. This might be especially important for patients with a low rectal tumor, as the risk of low anterior resection syndrome is higher in these patients receiving a primary anastomosis. Additionally, the reduction in permanent stoma rate may also have an effect on healthcare costs, as costs associated with daily care of the stoma, stoma-related complications, readmissions, and reinterventions occur more often in patients having a permanent stoma. Both aspects should be taken into account in future analyses.
Certain limitations should be taken into account when interpreting the results. First, this is a retrospective study, hence bias might be present. We tried to control for confounding by indication by performing a multivariable regression analysis, although residual confounding might still be present. Furthermore, TaTME is generally not used to perform an APR, both rather using the laparoscopic technique. As an effect permanent stoma rate could be underestimated, whereas primary anastomosis rate and functional anastomosis rate could be overestimated. To account for this, we excluded the patients undergoing an APR from the primary outcome. However, by excluding these patients from all the analyses, selection bias might be present as well. As patients with a low tumor might be offered a restorative procedure in a robot-assisted or TaTME center, this would not have been offered in a laparoscopic center, as the robot-assisted and transanal techniques are suggested to provide better overview in low rectal tumors, thereby enabling sphincter-saving surgery in more patients. Therefore, we presented data regarding all TME patients, LAR patients, and restorative LAR patients, stratified per technique (laparoscopic, robot-assisted, and transanal) and stratified per center (laparoscopic center, robot-assisted center, and transanal centers). Furthermore, stoma complications might be underestimated due to the retrospective nature of the study. Nevertheless, permanent stoma rate, readmissions, and reinterventions are generally well documented, and less prone to report bias.
Second, the difference in permanent stoma rate, which might be caused by difference in primary anastomosis rate, might not only be associated with the technique: surgeon-related or center-related preferences might play a part as well. Unfortunately, controlling for surgeons was not possible as we did not register this. Furthermore, controlling for centers in the multivariable regression analysis was not possible, as this would lead to multicollinearity. However, no large differences of the crude permanent stoma rates within L-TME, R-TME, and TaTME centers were observed.
Third, the present cohort consists of patients operated by experienced surgeons. Therefore, these results might not directly be extrapolated to all rectal cancer patients. Finally, we used the MRI-based definition for rectal cancer as proposed by D’Souza et al., thereby rectosigmoid tumors that might have been included if former definitions were used were excluded from this analysis. The inclusion of a relatively higher proportion of patients with a low rectal tumor could have led to a relatively high permanent stoma rate.
As this is the first study showing an association between surgical approach and permanent stoma rate, future prospective studies are necessary to confirm our results. Additionally, the impact on quality of life and costs should be investigated. Especially in low rectal tumors, more prospective data are necessary to assess risk of low anterior resection syndrome and its associated influence on quality of life and cost-effectivity in case of restorative rectal resection.
In conclusion, the robot-assisted and TaTME technique are associated with a lower permanent stoma rate compared to the laparoscopic technique in patients undergoing a LAR. This association might be an effect of the higher primary anastomosis rate in these two techniques.
Data availability
Data are available on reasonable request, this includes template data collection forms, data extracted from included studies, data used for analyses, and any other materials used in the review.
Code availability
Code is available on request.
References
Simillis C, Lal N, Thoukididou SN et al (2019) Open versus laparoscopic versus robotic versus transanal mesorectal excision for rectal cancer: a systematic review and network meta-analysis. Ann Surg 270(1):59–68. https://doi.org/10.1097/SLA.0000000000003227
Heald RJ, Ryall RDH (1986) Recurrence and survival after total mesorectal excision for rectal cancer. Lancet 327(8496):1479–1482. https://doi.org/10.1016/S0140-6736(86)91510-2
Heald RJ, Husband EM, Ryall RDH (1982) The mesorectum in rectal cancer surgery—the clue to pelvic recurrence? Br J Surg 69(10):613–616. https://doi.org/10.1002/bjs.1800691019
Matthiesen P, Hallböök O, Rutegard J, Simert G, Sjödahl R (2007) Defunctioning stoma reduces symptomatic anastomotic leakage after low anterior resection of the rectum for cancer. A randomized multicenter trial. Ann Surg 246(2):207–214. https://doi.org/10.1097/SLA.0b013e3180603024
Hüser N, Michalski CW, Erkan M et al (2008) Systematic review and meta-analysis of the role of defunctioning stoma in low rectal cancer surgery. Ann Surg 248(1):52–60. https://doi.org/10.1097/SLA.0b013e318176bf65
Pisarska M, Gajewska N, Małczak P et al (2018) Defunctioning ileostomy reduces leakage rate in rectal cancer surgery—systematic review and meta-analysis. Oncotarget 9(29):20816–20825. https://doi.org/10.18632/oncotarget.25015
Kim S, Kim MH, Oh JH et al (2020) Predictors of permanent stoma creation in patients with mid or low rectal cancer: results of a multicentre cohort study with preoperative evaluation of anal function. Color Dis 22(4):399–407. https://doi.org/10.1111/codi.14898
den Dulk M, Smit M, Peeters KC et al (2007) A multivariate analysis of limiting factors for stoma reversal in patients with rectal cancer entered into the total mesorectal excision (TME) trial: a retrospective study. Lancet Oncol 8(4):297–303. https://doi.org/10.1016/S1470-2045(07)70047-5
Näsvall P, Dahlstrand U, Löwenmark T, Rutegård J, Gunnarsson U, Strigård K (2017) Quality of life in patients with a permanent stoma after rectal cancer surgery. Qual Life Res 26(1):55–64. https://doi.org/10.1007/s11136-016-1367-6
Gooszen AW, Geelkerken RH, Hermans J, Lagaay MB, Gooszen HG (2000) Quality of life with a temporary stoma: ileostomy vs. colostomy. Dis Colon Rectum 43(5):650–655. https://doi.org/10.1007/BF02235581
Tseng HC, Wang HH, Hsu YY, Weng WC (2004) Factors related to stress in outpatients with permanent colostomies. Kaohsiung J Med Sci 20(2):70–76. https://doi.org/10.1016/s1607-551x(09)70087-7
Shabbir J, Britton DC (2010) Stoma complications: a literature overview. Color Dis 12(10):958–964. https://doi.org/10.1111/j.1463-1318.2009.02006.x
Kwiatt M, Kawata M (2013) Avoidance and management of stomal complications. Clin Colon Rectal Surg 26(2):112–121. https://doi.org/10.1055/s-0033-1348050
Hol JC, Burghgraef TA, Rutgers MLW et al (2021) Comparison of laparoscopic versus robot-assisted versus transanal total mesorectal excision surgery for rectal cancer: a retrospective propensity score-matched cohort study of short-term outcomes. Br J Surg. https://doi.org/10.1093/bjs/znab233
Olthof PB, Giesen LJX, Vijfvinkel TS, Roos D, Dekker JWT (2020) Transition from laparoscopic to robotic rectal resection: outcomes and learning curve of the initial 100 cases. Surg Endosc 1:3. https://doi.org/10.1007/s00464-020-07731-0
Burghgraef TA, Crolla RM, Verheijen PM, Fahim M, van Geloven A, Leijtens JW, Pronk A, Smits AB, Verdaasdonk EG, Consten EC (2022) Robot-assisted total mesorectal excision versus laparoscopic total mesorectal excision: a retrospective propensity score-matched cohort analysis in experienced centers. Dis Colon Rectum 65(2):218–27
Feng Q, Yuan W, Li T et al (2022) Robotic versus laparoscopic surgery for middle and low rectal cancer (REAL): short-term outcomes of a multicentre randomised controlled trial. Lancet Gastroenterol Hepatol 7(11):991–1004. https://doi.org/10.1016/S2468-1253(22)00248-5
Souza N, Tot Babberich MP, Hoore A, Tiret E, Xynos E, Beets-Tan RG, Nagtegaal ID, Blomqvist L, Holm T, Glimelius B, Lacy A (2019) Definition of the rectum: an international, expert-based Delphi Consensus. Ann Surg 270(6):955–959
von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP (2008) The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 61(4):344–349. https://doi.org/10.1016/j.jclinepi.2007.11.008
Rahbari NN, Weitz J, Hohenberger W et al (2010) Definition and grading of anastomotic leakage following anterior resection of the rectum : a proposal by the International Study Group of Rectal Cancer. Surgery 147:339–351. https://doi.org/10.1016/j.surg.2009.10.012
Landelijke werkgroep Gastro Intestinale Tumoren (2019) Richtlijn colorectaal carcinoom 4.0. https://www.oncoline.nl/colorectaalcarcinoom. Accessed 13 Jan 2020
Seo SI, Yu CS, Kim GS et al (2013) Characteristics and risk factors associated with permanent stomas after sphincter-saving resection for rectal cancer. World J Surg 37(10):2490–2496. https://doi.org/10.1007/s00268-013-2145-z
Lee CM, Huh JW, Park YA et al (2015) Risk factors of permanent stomas in patients with rectal cancer after low anterior resection with temporary stomas. Yonsei Med J 56(2):447–453. https://doi.org/10.3349/ymj.2015.56.2.447
Miura T, Sakamoto Y, Morohashi H, Yoshida T, Sato K, Hakamada K (2018) Risk factor for permanent stoma and incontinence quality of life after sphincter-preserving surgery for low rectal cancer without a diverting stoma. Ann Gastroenterol Surg 2(1):79–86. https://doi.org/10.1002/ags3.12033
Kim MJ, Kim YS, Park SC et al (2016) Risk factors for permanent stoma after rectal cancer surgery with temporary ileostomy. Surgery 159(3):721–727. https://doi.org/10.1016/j.surg.2015.09.011
Gadan S, Floodeen H, Lindgren R, Rutegård M, Matthiessen P (2020) What is the risk of permanent stoma beyond 5 years after low anterior resection for rectal cancer? A 15-year follow-up of a randomized trial. Color Dis 22(12):2098–2104. https://doi.org/10.1111/codi.15364
Shiraishi T, Nishizawa Y, Ikeda K, Tsukada Y, Sasaki T, Ito M (2020) Risk factors for parastomal hernia of loop stoma and relationships with other stoma complications in laparoscopic surgery era. BMC Surg. https://doi.org/10.1186/s12893-020-00802-y
Krishnamurty DM, Blatnik J, Mutch M (2017) Stoma complications. Clin Colon Rectal Surg 30(3):193–200. https://doi.org/10.1055/s-0037-1598160
Koc U, Karaman K, Gomceli I et al (2017) A retrospective analysis of factors affecting early stoma complications. Ostomy Wound Manag 63(1):28–32
Back E, Häggström J, Holmgren K et al (2021) Permanent stoma rates after anterior resection for rectal cancer: risk prediction scoring using preoperative variables. Br J Surg 108(11):1388–1395. https://doi.org/10.1093/bjs/znab260
Greijdanus NG, Wienholts K, Ubels S et al (2023) Stoma-free survival after rectal cancer resection with anastomotic leakage. Ann Surg. https://doi.org/10.1097/SLA.0000000000006043
Malik TAM, Lee MJ, Harikrishnan AB (2018) The incidence of stoma related morbidity—a systematic review of randomised controlled trials. Ann R Coll Surg Engl 100(7):501–508. https://doi.org/10.1308/rcsann.2018.0126
Nastro P, Knowles CH, McGrath A, Heyman B, Porrett TRC, Lunniss PJ (2010) Complications of intestinal stomas. Br J Surg 97(12):1885–1889. https://doi.org/10.1002/bjs.7259
Hazen SJA, Vogel I, Borstlap WAA et al (2021) Long-term stoma-related reinterventions after anterior resection for rectal cancer with or without anastomosis: population data from the Dutch snapshot study. Tech Coloproctol. https://doi.org/10.1007/s10151-021-02543-3
Lawday S, Flamey N, Fowler GE et al (2021) Quality of life in restorative versus non-restorative resections for rectal cancer: systematic review. BJS Open. https://doi.org/10.1093/bjsopen/zrab101
Pachler J, Wille-Jørgensen P (2012) Quality of life after rectal resection for cancer, with or without permanent colostomy. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.cd004323.pub4
Orsini RG, Thong MSY, Van De Poll-Franse LV et al (2013) Quality of life of older rectal cancer patients is not impaired by a permanent stoma. Eur J Surg Oncol 39(2):164–170. https://doi.org/10.1016/j.ejso.2012.10.005
Feddern ML, Emmertsen KJ, Laurberg S (2019) Quality of life with or without sphincter preservation for rectal cancer. Colorectal Dis 21(9):1051–1057. https://doi.org/10.1111/CODI.14684
Acknowledgements
On behalf of the MIRECA (Minimal invasive rectal cancer surgery) group, R.M.P.H. Crolla; A.A.W. van Geloven, MD, PhD; J.C. Hol, MD; J.W.A. Leijtens, MD, PhD; F. Polat, MD, PhD; A. Pronk, MD, PhD; M.L. Rutgers, MD; C. Sietses, MD, PhD; A.B. Smits, MD, PhD; J.B. Tuynman, MD, PhD; E.G.G. Verdaasdonk, MD, PhD; and P.M. Verheijen, MD, PhD are gratefully acknowledged.
Author information
Authors and Affiliations
Consortia
Contributions
TAB, ECJC, RH, MB, JCH, and RTJG contributed to the study conception and design. The data retrieval was performed by TAB, RTJG, and JCH. The data analysis was performed by TAB and MB. The first draft was written by TAB, and all authors critically revised the work.
Corresponding author
Ethics declarations
Disclosures
No funding was received for conducting this study. Esther C.J. Consten receives fees from Intuitive Surgical. Roel Hompes receives fees from Applied Medical. Thijs A. Burghgraef, Mark Broekman, Jeroen C. Hol, and Ritch T.J. Geitenbeek have no relevant financial or non-financial interests to disclose.
Ethical approval
Informed consent was deemed unnecessary according to the Dutch Medical Treatment Agreement Act. The medical ethical committee and local ethical committees of all hospitals gave approval for the study (MEC-U, AW19.023 W18.100).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Burghgraef, T.A., Geitenbeek, R.T.J., Broekman, M. et al. Permanent stoma rate and long-term stoma complications in laparoscopic, robot-assisted, and transanal total mesorectal excisions: a retrospective cohort study. Surg Endosc 38, 105–115 (2024). https://doi.org/10.1007/s00464-023-10517-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-023-10517-9