Abstract
Background
Viral transmission to healthcare providers during surgical procedures was a major concern at the outset of the COVID-19 pandemic. The presence of the severe acute respiratory disease syndrome coronavirus (SARS-CoV-2), the virus responsible for COVID-19, in the abdominal cavity as well as in other abdominal tissues which surgeons are exposed has been investigated in several studies. The aim of the present systematic review was to analyze if the virus can be identify in the abdominal cavity.
Methods
We performed a systematic review to identify relevant studies regarding the presence of SARS-CoV-2 in abdominal tissues or fluids. Number of patients included as well as patient’s characteristics, type of procedures, samples and number of positive samples were analyzed.
Results
A total of 36 studies were included (18 case series and 18 case reports). There were 357 samples for detection of SARS-CoV-2, obtained from 295 individuals. A total of 21 samples tested positive for SARS-CoV-2 (5.9%). Positive samples were more frequently encountered in patients with severe COVID-19 (37.5% vs 3.8%, p < 0.001). No health-care provider related infections were reported.
Conclusion
Although a rare occurrence, SARS-CoV-2 can be found in the abdominal tissues and fluids. It seems that the presence of the virus in the abdominal tissues or fluids is more likely in patients with severe disease. Protective measures should be employed in the operating room to protect the staff when operating patients with COVID-19.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The coronavirus disease 2019 (COVID-19) pandemic had an unprecedented impact in the world [1]. Numerous changes were implemented across the healthcare system to mitigate the risk of viral spread to healthcare workers while providing care to those who were infected. Surgical care was deeply affected as it was not exempt to the evolving adjustments produced by the pandemic, including the early deployment of surgeons to non-surgical responsibilities where much help was needed [2,3,4]. In addition, millions of elective surgeries were canceled to both safeguard the needed resources for the incoming influx of infected patients, as well to protect the healthcare providers from a potential infection. [2]
Based on extrapolated data from other viruses, there was a fear that surgeons could be exposed to infective levels of virus while performing surgery [5, 6]. Some societies published recommendations cautioning against the use of laparoscopy in COVID-positive patients as this was considered to be a potential aerosolizing procedure [5, 6]. The hypothesis was that if the virus were present in the abdominal tissues, it could be aerosolized and travel along with the insufflation gas or surgical plume during laparoscopic port insertion and removal, port venting, during instrument exchanges or specimen extraction, and hence transmitted to the operating room staff. However, as previously mentioned, for a potential transmission, the virus would need to be present in the abdominal cavity. As such, several groups investigated the presence of the COVID-19 virus in the abdominal cavity, as well as in other abdominal tissues to which surgeons are exposed. [7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29]
Nevertheless, the results were conflicting, in addition to being based just on case reports and case series [7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43]. Early during the pandemic, a systematic review attempted to clarify the issue by pooling the available data [44]. However, they could not support the hypothesis that the COVID-19 virus could be aerosolized in the operating room, and the majority of publications analyzed for inclusion were case reports. Since then, more studies have been published in this matter. As the world recovers from the COVID-19 pandemic we must be prepare for the ongoing emergence of new variants and future pandemics. Thus, the objective of the present study was to study the presence of the COVID-19 virus in the abdominal tissues and fluids.
Methods
Study design
A systematic literature search to identify relevant studies regarding the presence of severe acute respiratory disease syndrome coronavirus (SARS-CoV-2), the virus responsible for COVID-19, in abdominal tissues or fluids. The query was performed using the following databases: PubMed, Web of Science, EMBASE and Cochrane Central Library. It was restricted to articles in English and Spanish languages and was not time limited. References from previous reviews, as well as references from relevant primary studies were manually searched to identify any additional studies. The search was completed on March 1st, 2023. The systematic review was conducted in conformity with the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines and was registered in the prospective international register of systematic reviews (PROSPERO: CRD42023408176). [45]
Search strategy
Medical subject heading (MeSH) terms to search was broad to encompass articles related to the identification of SARS-CoV-2 RNA in samples of abdominal tissues or fluids in patients with SARS-CoV-2 infection. Therefore, the terms were as follow: “SARS-COV-2”, “abdominal tissue*”, “abdominal fluid*”, “peritoneal fluid*”, “biologic* fluid*”, “swab test”, “bile”, “biliary”, and “surgery”. All identified abstracts were assessed for inclusion or exclusion by 2 independent evaluators (G.R-V. and G.P-B). There was no disagreement between evaluators that required the participation of a third investigator. Studies meeting the inclusion criteria were retrieved and the full texts reviewed.
Study selection
Observational studies (retrospective or prospective cohorts, case series, and case reports) that included patients with SARS-CoV-2 infection who underwent any abdominal procedure and in which samples of abdominal tissues or fluids were taken for qualitative detection of SARS-CoV-2 RNA were included in this systematic review.
Data extraction
Information extracted from eligible studies included basic study data (last name of the first author, country, design, sample size), demographic data (gender, age), SARS-CoV-2 infection status and symptoms (diagnostic test status, respiratory symptoms, radiologic signs of pneumonia, severity, length of disease), abdominal procedure parameters (type of procedure – surgery, paracentesis, dialysis, endoscopic procedures), and sample analysis (type, performed test for detection of SARS-CoV-2, positive status and timing with the surgery). Severe COVID-19 infection was defined as the patient requiring invasive respiratory support, intensive care unit admission or death [46]. Data were extracted and verified by three independent investigators (G.R-V, G.P-B, and M.A.Z.).
Analysis
Data within individual studies was extracted to fields within an Excel (Microsoft, Redmond, WA, USA) database. Data manipulation and analysis was performed using SAS v9.4 (SAS, Cary, NC, USA). The positivity between patients with severe and non-severe disease was analyzed with Chi-square, and statistical significance was defined as p < 0.05.
Results
Search results and study characteristics
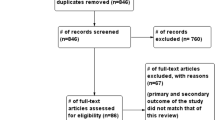
A total of 335 studies (G.P-B. and G.R-V.) were identified from the previously described search strategy. After duplicates were removed, the titles and full abstracts of 259 studies were evaluated, from which only 45 were assessed for eligibility. The full text of these 45 articles were reviewed, and 36 were identified for inclusion (Fig. 1). From the included studies, 18 were case series (Table 1), and 18 were case reports (Table 2).
Descriptive analysis
A total of 295 patients were included, of whom 76% were female (n = 224). Age range was between 12 and 95 years. At least 141 (47.8%) patients were positive for SARS-CoV-2 at the moment of sample collection, in whom respiratory symptoms were registered in 109 (36.9%), and radiologic findings were described in 46 (15.6%). Severe COVID-19 infection, as previously defined was registered in 32 patients (10.8%).
Abdominal sample characteristics
Reverse transcriptase-polymerase chain reaction (RT-PCR) was the test used in all of the cases. Collection time of the abdominal sample from the presence of symptoms ranged between 0 and 63 days. Samples for detection of SARS-CoV-2 were obtained from direct swab of the peritoneum or peritoneal fluid during surgery or paracentesis in 259 individuals, from peritoneal dialysis in 43, from smoke during surgery in 25, from bile or other gastrointestinal fluid in 13, and 17 from other tissues (including omentum, subcutaneous tissue, and hollow organs). Out of 357 total samples, 21 were reported to be positive (5.9%).
From all positive samples, 12 were in patients with severe COVID-19 infection (57.1%). Patients with severe COVID-19 infection were more likely to have positive samples compared to patients without severe infection (37.5% vs 3.8%, p < 0.001). Description of SARS-CoV-2 positive samples from abdominal sources is shown in Table 3 There were no healthcare provider infections reported across the included studies.
Discussion
As the world emerges from the COVID-19 pandemic there is opportunity to re-examine and collate the data collected during the pandemic to draw more concrete conclusions and prepare for potential future pandemics. As we are likely to experience new variants and future pandemics, surgeons need to be prepared and understand the potential risks for viral exposure and infection in the operating room. A key component to comprehend the potential for COVID-19 transmission during abdominal operations is to delimit the presence of the virus in the abdominal cavity. As there are conflicting results coming from case reports and small case series, the purpose of this study was to assess all the available data. We found that although a rare occurrence, SARS-CoV-2 can be isolated from abdominal tissues and fluids. While this study includes a small number of asymptomatic patients, it is noted that positive intra-peritoneal samples were noted among patients without disease symptoms.
Our study has some strengths and fills in a gap in the literature which are worth noting. First, more studies have been published since the previous systematic review in this topic was published, which allows to expand on the prior conclusions [44]. In addition, compared to the recently published guidelines by the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) for the use of laparoscopy in the era of COVID-19, our search strategy included the presence of SARS-CoV-2 in different specimens rather than just surgical plume. [5]
Our results support the recommendation by SAGES in which protective measures should be employed when operating on patients with COVID-19 for all cases regardless of the approach [5]. Nevertheless, it should be pointed out that even though SARS-CoV-2 has been found in the abdominal cavity by RT-PCR, the potential of these particles to infect has never been demonstrated [47]. In fact, none of the studies included in our review reported health care associated infections to the operating room staff. It is important to highlight that the type and extent of personal protective equipment or other isolation measures employed in the included studies are not known. Infections among healthcare workers may also be difficult to attribute to a specific exposure, as any worker in that environment presumably could have multiple potential opportunities for viral exposure throughout their routine course of work. Thus, a definitive statement regarding transmissibility of the detected viral loads is not possible and future studies should focus on the potential infectivity of viral particles found in the abdominal cavity.
Furthermore, the source of SARS-CoV-2 found in the peritoneum has also been investigated. Some reports have considered positive samples to be due to contamination by feces, bile or blood, which are well known to convey viral particles [36, 38, 44, 48,49,50]. Although some of the included cases in our review could be explained by contamination, the virus was also found in the dialysate effluent of peritoneal dialysis which likely is not prone to this contamination. This suggests that peritoneal viral translocation could occur in the absence of inflammation, but also be potentiated in the setting of inflammation [49]. Another important consideration in this regard is the correlation we found between severe COVID-19 disease and positive samples. Fabbri et al. have previously suggested the possibility that severe disease would translate to higher viral load and hence the increased possibility of translocation to the abdominal cavity [32]. Regardless of the mechanism responsible for the presence of the virus in the abdominal cavity, the data suggest that surgeons could be exposed to viral particles when operating.
There are important limitations of the current study. First and most important, although we have evaluated multiple studies, the data comes from case series and case reports which are prone to selection bias. Also, there was significant heterogeneity of RT-PCR used, including varying promoter sequences and pooling or non-pooling techniques, the sampling site, and timing between COVID-19 diagnosis and specimen collection. In addition, these studies were completed during varying phases of the pandemic and likely include multiple SARS-COV2 variants and subvariants and whether a specific subvariant is more likely to be found in intra-abdominal tissues is not known. All of these factors in addition to the fact that RT-PCR may not have been validated for viral RNA detection from samples obtained from the abdominal cavity has been previously acknowledged by Fabbri et al. [49] Specifically, there may be a high false positive rate of RT-PCR, and nucleic acid amplification tests such as RT-PCR may remain positive for weeks to months after infection. Moreover, some tests designed and approved for screening and others for confirmatory diagnostic testing, which necessarily have different performance characteristics and expected positivity rates.
This study supports the assertion that SARS-CoV-2 can be identified from abdominal tissues and fluids that would be normally encountered during surgery. It seems that the presence of the virus in the abdominal tissues and fluids is more likely in patients with severe disease. Protective measures are needed in the operating room to protect the staff when operating patients with COVID-19.
References
Nkengasong JN (2021) COVID-19: unprecedented but expected. Nat Med 27(3):364–364. https://doi.org/10.1038/s41591-021-01269-x
Kibbe MR (2020) Surgery and COVID-19. JAMA 324(12):1151. https://doi.org/10.1001/jama.2020.15191
In H, Muscarella P, Moran-Atkin E, Michler RE, Melvin WS (2020) Reflections on the coronavirus disease 2019 (COVID-19) epidemic: The first 30 days in one of New York’s largest academic departments of surgery. Surgery 168(2):212–214. https://doi.org/10.1016/j.surg.2020.05.008
Beninato T, Laird AM, Graves CE et al (2022) Impact of the COVID-19 pandemic on the practice of endocrine surgery. Am J Surg 223(4):670–675. https://doi.org/10.1016/j.amjsurg.2021.07.009
Collings AT, Jeyarajah DR, Hanna NM et al (2022) SAGES 2022 guidelines regarding the use of laparoscopy in the era of COVID-19. Surg Endosc 36(5):2723–2733. https://doi.org/10.1007/s00464-022-09133-w
Francis N, Dort J, Cho E et al (2020) SAGES and EAES recommendations for minimally invasive surgery during COVID-19 pandemic. Surg Endosc 34(6):2327–2331. https://doi.org/10.1007/s00464-020-07565-w
Romero-Velez G, Pereira X, Zenilman A, Camacho D (2020) SARS-Cov-2 Was not found in the peritoneal fluid of an asymptomatic patient undergoing laparoscopic appendectomy. Surg Laparosc Endosc Percutaneous Techn 30(6):e43–e45. https://doi.org/10.1097/SLE.0000000000000837
Barberis A, Rutigliani M, Belli F, Ciferri E, Mori M, Filauro M (2020) SARS-Cov-2 in peritoneal fluid: an important finding in the Covid-19 pandemic. Br J Surg 107(10):e376–e376. https://doi.org/10.1002/bjs.11816
Passarelli VC, Perosa AH, de Souza Luna LK et al (2020) Detected SARS-CoV-2 in ascitic fluid followed by cryptococcemia: a case report. SN Compr Clin Med 2(11):2414–2418. https://doi.org/10.1007/s42399-020-00574-9
Scutari R, Piermatteo L, Manuelli MC et al (2020) Long-Term SARS-CoV-2 infection associated with viral dissemination in different body fluids including bile in two patients with acute cholecystitis. Life 10(11):302. https://doi.org/10.3390/life10110302
Agnes A, La Greca A, Tirelli F, Papa V (2020) Duodenal perforation in a SARS-CoV-2-positive patient with negative PCR results for SARS-CoV-2 in the peritoneal fluid. Eur Rev Med Pharmacol Sci 24(23):12516–12521
Coccolini F, Tartaglia D, Puglisi A et al (2020) SARS-CoV-2 Is present in peritoneal fluid in COVID-19 patients. Ann Surg 272(3):e240–e242. https://doi.org/10.1097/SLA.0000000000004030
Seeliger B, Philouze G, Benotmane I, Mutter D, Pessaux P (2020) Is the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) present intraperitoneally in patients with coronavirus disease 2019 (COVID-19) infection undergoing emergency operations? Surgery 168(2):220–221. https://doi.org/10.1016/j.surg.2020.05.033
Flemming S, Hankir M, Hering I et al (2020) Abdominal fluid samples (negative for SARS-CoV-2) from a critically unwell patient with respiratory COVID-19. Br J Surg 107(8):e259–e260. https://doi.org/10.1002/bjs.11713
Han D, Fang Q, Wang X (2021) SARS-CoV-2 was found in the bile juice from a patient with severe COVID-19. J Med Virol 93(1):102–104. https://doi.org/10.1002/jmv.26169
Candellier A, Scohy A, Gillet N et al (2020) Absence of SARS-CoV-2 in the effluent of peritoneal dialysis patients. Perit Dial Int 40(5):499–503. https://doi.org/10.1177/0896860820953061
El Shamy O, Vassalotti JA, Sharma S et al (2020) Coronavirus disease 2019 (COVID-19) hospitalized patients with acute kidney injury treated with acute peritoneal dialysis do not have infectious peritoneal dialysis effluent. Kidney Int 98(3):782. https://doi.org/10.1016/j.kint.2020.06.012
Gaba S, Kampalli G, Goyal K et al (2020) No Traces of SARS-CoV-2 In wounds of COVID-19 positive patients: a pilot study. Indian J Plast Surg 53(03):399–401. https://doi.org/10.1055/s-0040-1718852
Safari S, Keyvani H, Malekpour Alamdari N et al (2020) Abdominal surgery in patients with COVID-19: detection of SARS-CoV-2 in abdominal and adipose tissues. Ann Surg 272(3):e253–e256. https://doi.org/10.1097/SLA.0000000000004165
Balaphas A, Gkoufa K, Meyer J et al (2020) COVID-19 can mimic acute cholecystitis and is associated with the presence of viral RNA in the gallbladder wall. J Hepatol 73(6):1566–1568. https://doi.org/10.1016/j.jhep.2020.08.020
Ahmad S, Ahmed RN, Jani P, Ullah M, Aboulgheit H (2020) SARS–CoV-2 isolation from an appendix. J Surg Case Rep. https://doi.org/10.1093/jscr/rjaa245
Culver A, Arbelot C, Bechis C, Cassir N, Leone M (2020) First description of SARS-CoV-2 in ascites. IDCases. https://doi.org/10.1016/j.idcr.2020.e00836
Ngaserin SHN, Koh FH, Ong BC, Chew MH (2020) COVID-19 not detected in peritoneal fluid: a case of laparoscopic appendicectomy for acute appendicitis in a COVID-19-infected patient. Langenbecks Arch Surg 405(3):353–355. https://doi.org/10.1007/s00423-020-01891-2
Kabir T, Ngaserin S, Koh FH, Huang J, Ong BC, Chew MH (2020) The COVID-19 conundrum: SARS-CoV-2 is not present in bile. Br J Surg 107(10):e381–e381. https://doi.org/10.1002/bjs.11820
Liao Y, Wang B, Wang J, Shu J, Zhou W, Zhang H (2020) SARS-CoV-2 in the bile of a patient with COVID-19-associated gallbladder disease. Endoscopy 52(12):1148–1148. https://doi.org/10.1055/a-1290-7446
Mattone E, Sofia M, Schembari E et al (2020) Acute acalculous cholecystitis on a COVID-19 patient: a case report. Ann Med Surg 58:73–75. https://doi.org/10.1016/j.amsu.2020.08.027
Sadioglu RE, Aktar M, Gulten E et al (2020) Does SARS-CoV-2 reach peritoneal effluent? Perit Dial Int 40(5):520–521. https://doi.org/10.1177/0896860820947331
Vischini G, D’Alonzo S, Grandaliano G, D’Ascenzo FM (2020) SARS-CoV-2 in the peritoneal waste in a patient treated with peritoneal dialysis. Kidney Int 98(1):237–238. https://doi.org/10.1016/j.kint.2020.05.005
Ying M, Lu B, Pan J et al (2020) COVID-19 with acute cholecystitis: a case report. BMC Infect Dis 20(1):437. https://doi.org/10.1186/s12879-020-05164-7
AlAradi J, AlHarmi RAR, AlKooheji M, Almahari SA, Isa MA, AlMarzooq R (2021) SARS-CoV-2 in peritoneal swabs from asymptomatic patients undergoing emergency abdominal surgery. J Surg Case Rep. https://doi.org/10.1093/jscr/rjab116
Bogani G, Ditto A, De Cecco L et al (2021) Transmission of SARS-CoV-2 in surgical smoke during laparoscopy: a prospective, proof-of-concept study. J Minim Invasive Gynecol 28(8):1519–1525. https://doi.org/10.1016/j.jmig.2020.12.026
Fabbri N, Pesce A, Ussia A et al (2022) Swab test in biological fluids as predictor of COVID-19 transmission risk during surgery: a prospective cross-sectional study from an Italian COVID center. BMC Surg 22(1):119. https://doi.org/10.1186/s12893-022-01571-6
Jakimiuk AJ, Januszewski M, Santor-Zaczynska M et al (2021) Absence of SARS-CoV-2 RNA in peritoneal fluid during surgery in pregnant women who are COVID-19 positive. J Minim Invasive Gynecol 28(12):2047–2051. https://doi.org/10.1016/j.jmig.2021.06.006
Jones D, Faluyi D, Hamilton S et al (2021) A prospective study to identify rates of SARS-CoV-2 virus in the peritoneum and lower genital tract of patients having surgery: an observational study. J Minim Invasive Gynecol 28(9):1633–1636. https://doi.org/10.1016/j.jmig.2021.02.006
Pani E, Collini L, Naselli A et al (2022) SARS-Cov-2 in peritoneal fluid of two children with COVID-19: a rare finding. J Paediatr Child Health 58(4):702–704. https://doi.org/10.1111/jpc.15610
Romero-Velez G, Rodriguez Quintero JH, Pereira X, Nussbaum JE, McAuliffe JC (2021) SARS-CoV-2 during abdominal operations: are surgeons at risk? Surg Laparosc Endosc Percutaneous Tech 31(6):674–678. https://doi.org/10.1097/SLE.0000000000000971
Suárez M, Rodríguez D, Morales D, Arellano E, Hernández LAMT (2021) RT-PCR for SARS-CoV-2 in dialysis effluent on four patients from an ambulatory peritoneal dialysis program in Mexico City. Nefrologia (Engl Ed). https://doi.org/10.1016/j.nefro.2021.05.006
Tartaglia D, Barberis A, Coccolini F, Pistello M, Rutigliani M, Chiarugi M (2022) Positive peritoneal swab in SARS-CoV-2 patients undergoing abdominal emergency surgery: effect or cause? Infection 50(4):989–993. https://doi.org/10.1007/s15010-022-01785-z
Vimalachandran D, Jones RP, Dickson E et al (2023) SARS-CoV-2 in the abdomen or pelvis: safe surgery study. Br J Surg 110(3):306–309. https://doi.org/10.1093/bjs/znac297
Wang X, Patel A, Tisdale L et al (2021) SARS-CoV-2 in spent dialysate from chronic peritoneal dialysis patients with COVID-19. Kidney360 2(1):86–89. https://doi.org/10.34067/KID.0006102020
D’Introno A, Gatti P, Manca G, D’Amuri A, Minniti S, Ciracì E (2022) Acute acalculous cholecystitis as an early manifestation of COVID-19: case report and literature review. Acta Biomedica Atenei Parmensis. https://doi.org/10.23750/abm.v93iS1.12760
Hong X, He J, Li P et al (2021) Evidence of SARS-CoV-2 infection in gallbladder and aggravating cholecystitis to septic shock: a case report. Ann Transl Med 9(21):1631–1631. https://doi.org/10.21037/atm-21-4778
Mofti AH, Ghabashi FA, Sadagah MM et al (2021) Sclerosing encapsulating peritonitis following recovery from COVID-19 pneumonia. Cureus. https://doi.org/10.7759/cureus.19306
Cheruiyot I, Sehmi P, Ngure B et al (2021) Laparoscopic surgery during the COVID-19 pandemic: detection of SARS-COV-2 in abdominal tissues, fluids, and surgical smoke. Langenbecks Arch Surg 406(4):1007–1014. https://doi.org/10.1007/s00423-021-02142-8
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. https://doi.org/10.1371/journal.pmed.1000097
Berlin DA, Gulick RM, Martinez FJ (2020) Severe Covid-19. N Engl J Med 383(25):2451–2460. https://doi.org/10.1056/NEJMcp2009575
Seeliger B, Pessaux P (2021) Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) RNA in peritoneal fluid of coronavirus disease 2019 (COVID-19) patients—prevalence and significance. Surgery 169(6):1559–1560. https://doi.org/10.1016/j.surg.2021.02.025
Barone M, Ippoliti M, Mucilli F (2021) SARS-CoV-2 peritoneal positivity and emergency surgery: is there any putative predisposing factor? Updates Surg 73(4):1593–1595. https://doi.org/10.1007/s13304-021-01066-8
Fabbri N, Pesce A, Feo CV, Pizzicotti S (2021) Peritoneal swab test for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) patients in abdominal surgery: is it a reliable practice? Surgery 169(6):1558–1559. https://doi.org/10.1016/j.surg.2021.01.044
Zhao Y, Ren X, Lu J et al (2023) Mechanistic insight of SARS-CoV-2 infection using human hepatobiliary organoids. Gut 72(1):216–218. https://doi.org/10.1136/gutjnl-2021-326617
Funding
There was no funding for this project.
Author information
Authors and Affiliations
Contributions
Study Concept and design: GRV, GPLB, MK. Acquisition, analysis and interpretation: GRV, GPLB, MAZ. Drafting the manuscript: All authors. Critical revision of the manuscript for important intellectual content: All authors. Statistical analysis: GPLB. Administrative, technical or material support: MAZ, RC, SN. Study supervision: MK
Corresponding author
Ethics declarations
Disclosures
Gustavo Romero-Velez, Guillermo Ponce de Leon-Ballesteros, Maryam Al Zubaidi, Juan S. Barajas-Gamboa, Jerry Dang, Ricard Corcelles, Andrew Strong, Salvador Navarrete: No conflicts of interest or financial ties to disclose. Mathew Kroh: Consultant for Levita Magnetics; research funding from Cook Biotech, Medtronic and Pacira Pharmaceuticals.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Romero-Velez, G., Ponce de Leon-Ballesteros, G., Al Zubaidi, M. et al. Presence of SARS-CoV-2 in abdominal tissues and biologic fluids during abdominal surgery: a systematic review. Surg Endosc 37, 5011–5021 (2023). https://doi.org/10.1007/s00464-023-10130-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-023-10130-w