Abstract
Background
Use of macroporous synthetic mesh in contaminated ventral hernia repair has become more frequent. The objective of this study is to compare the outcomes of ventral incisional hernia repair with permanent synthetic mesh in contaminated fields to those in a clean field.
Methods
The Abdominal Core Health Quality Collaborative registry, a prospectively updated longitudinal hernia-specific national database, was retrospectively queried for adults who underwent open ventral incisional hernia repair using light or medium-weight synthetic mesh and classified as clean (CDC Class I) or contaminated (CDC Class II/III). Univariate analysis was used to compare demographic information, hernia characteristics, and operative details. Odds ratios (OR) were calculated using multivariable logistic regression for the primary outcome of 30-day surgical site infection (SSI) and secondary outcomes of 30-day surgical site occurrence (SSO), SSO requiring procedural intervention (SSO-PI), and clinical recurrence at one year.
Results
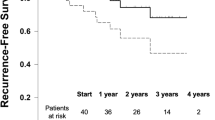
7219 cases met criteria for inclusion; 13.2% of these were contaminated. 83.4% of patients had follow-up data at 30 days and 20.8% at 1 year. The adjusted OR for 30-day SSI in contaminated fields compared to clean was 2.603 (95% CI 1.959–3.459). OR for 30-day SSO was 1.275 (95% CI 1.017–1.600) and 2.355 (95%CI 1.817–3.053) for 30-day SSO-PI. OR for recurrence at one year was 1.489 (95%CI 0.892–2.487). Contaminated cases had higher rates of mesh infection (3.9% vs 0.8%, p < 0.001) and mesh removal (7.3 vs 2.5%, p < 0.001) at 1 year.
Conclusions
After adjusting for baseline differences, patients undergoing ventral incisional hernia repair using light or midweight synthetic mesh in contaminated fields have higher odds of 30-day SSI, SSO, and SSO-PI than those performed in clean wounds. The odds of recurrence did not statistically differ and further studies with long-term outcomes are needed to better evaluate the best treatment options for this patient population.


Similar content being viewed by others
References
Choi JJ, Palaniappa NC, Dallas KB, Rudich TB, Colon MJ, Divino CM (2012) Use of mesh during ventral hernia repair in clean-contaminated and contaminated cases: outcomes of 33,832 cases. Ann Surg 255(1):176–180
Fischer JP, Wink JD, Tuggle CT, Nelson JA, Kovach SJ (2015) Wound risk assessment in ventral hernia repair: generation and internal validation of a risk stratification system using the ACS-NSQIP. Hernia 19(1):103–111
Holton LH 3rd, Kim D, Silverman RP, Rodriguez ED, Singh N, Goldberg NH (2005) Human acellular dermal matrix for repair of abdominal wall defects: review of clinical experience and experimental data. J Long Term Eff Med Implants 15(5):547–558
Ventral Hernia Working Group, Breuing K, Butler CE, et al (2010) Incisional ventral hernias: review of the literature and recommendations regarding the grading and technique of repair. Surgery 148(3):544–558.
Cole WC, Balent EM, Masella PC, Kajiura LN, Matsumoto KW, Pierce LM (2015) An experimental comparison of the effects of bacterial colonization on biologic and synthetic meshes. Hernia 19(2):197–205
Blatnik JA, Krpata DM, Jacobs MR, Gao Y, Novitsky YW, Rosen MJ (2012) In vivo analysis of the morphologic characteristics of synthetic mesh to resist MRSA adherence. J Gastrointest Surg 16(11):2139–2144
Krpata DM, Stein SL, Eston M et al (2013) Outcomes of simultaneous large complex abdominal wall reconstruction and enterocutaneous fistula takedown. Am J Surg 205(3):354–358 (discussion 358-359)
Carbonell AM, Criss CN, Cobb WS, Novitsky YW, Rosen MJ (2013) Outcomes of synthetic mesh in contaminated ventral hernia repairs. J Am Coll Surg 217(6):991–998
Kelly ME, Behrman SW (2002) The safety and efficacy of prosthetic hernia repair in clean-contaminated and contaminated wounds. Am Surg 68(6):524–528 (discussion 528-529)
Bondre IL, Holihan JL, Askenasy EP et al (2016) Suture, synthetic, or biologic in contaminated ventral hernia repair. J Surg Res 200(2):488–494
Chamieh J, Tan WH, Ramirez R, Nohra E, Apakama C, Symons W (2017) Synthetic versus biologic mesh in single-stage repair of complex abdominal wall defects in a contaminated field. Surg Infect (Larchmt) 18(2):112–118
Fischer JP, Basta MN, Krishnan NM, Wink JD, Kovach SJ (2016) A cost-utility assessment of mesh selection in clean-contaminated Ventral Hernia Repair. Plast Reconstr Surg 137(2):647–659
Harris HW, Primus F, Young C et al (2021) Preventing Recurrence in Clean and Contaminated Hernias Using Biologic Versus Synthetic Mesh in Ventral Hernia Repair: The PRICE Randomized Clinical Trial. Ann Surg 273(4):648–655
Hodgkinson JD, Maeda Y, Leo CA, Warusavitarne J, Vaizey CJ (2017) Complex abdominal wall reconstruction in the setting of active infection and contamination: a systematic review of hernia and fistula recurrence rates. Colorectal Dis 19(4):319–330
Majumder A, Winder JS, Wen Y, Pauli EM, Belyansky I, Novitsky YW (2016) Comparative analysis of biologic versus synthetic mesh outcomes in contaminated hernia repairs. Surgery 160(4):828–838
Warren J, Desai SS, Boswell ND et al (2020) Safety and efficacy of synthetic mesh for ventral hernia repair in a contaminated field. J Am Coll Surg 230(4):405–413
Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR (1999) Guideline for prevention of surgical site infection, 1999. Centers for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee. Am J Infect Control 27(2):97–132 (quiz 133-134; discussion 196)
Cobb WS, Carbonell AM, Kalbaugh CL, Jones Y, Lokey JS (2009) Infection risk of open placement of intraperitoneal composite mesh. Am Surg 75(9):762–767 (discussion 767-768)
Kercher KW, Sing RF, Matthews BD, Heniford BT (2002) Successful salvage of infected PTFE mesh after ventral hernia repair. Ostomy Wound Manage 48(10):40–42, 44–45.
Krpata DM, Blatnik JA, Novitsky YW, Rosen MJ (2013) Evaluation of high-risk, comorbid patients undergoing open ventral hernia repair with synthetic mesh. Surgery 153(1):120–125
Fafaj A, Tastaldi L, Alkhatib H et al (2021) Management of ventral hernia defect during enterocutaneous fistula takedown: practice patterns and short-term outcomes from the Abdominal Core Health Quality Collaborative. Hernia 25(4):1013–1020
Rosen MJ, Krpata DM, Petro CC et al (2022) Biologic vs synthetic mesh for single-stage repair of contaminated ventral hernias: a randomized clinical trial. JAMA Surg 157(4):293
Levy SM, Lally KP, Blakely ML et al (2015) Surgical wound misclassification: a multicenter evaluation. J Am Coll Surg 220(3):323–329
Huntington CR, Cox TC, Blair LJ et al (2016) Biologic mesh in ventral hernia repair: outcomes, recurrence, and charge analysis. Surgery 160(6):1517–1527
Shao JM, Ayuso SA, Deerenberg EB et al (2022) Biologic mesh is non-inferior to synthetic mesh in CDC class 1 & 2 open abdominal wall reconstruction. Am J Surg 223(2):375–379
Onyekwelu I, Yakkanti R, Protzer L, Pinkston CM, Tucker C, Seligson D (2017) Surgical wound classification and surgical site infections in the orthopaedic patient. J Am Acad Orthop Surg Glob Res Rev 1(3):e022
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Richard A Pierce: Receives research support from Intuitive Surgical Solutions, Cook Biotech, and Bard/Davol. Monica E Polcz, Molly A Olson, Joseph Blankush, Meredith C Duke, Joseph Broucek Joel F Bradley, III: no disclosures.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Polcz, M.E., Pierce, R.A., Olson, M.A. et al. Outcomes of light and midweight synthetic mesh use in clean-contaminated and contaminated ventral incisional hernia repair: an ACHQC comparative analysis. Surg Endosc 37, 5583–5590 (2023). https://doi.org/10.1007/s00464-022-09739-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-022-09739-0