Abstract
Background
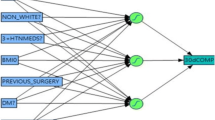
Postoperative gastrointestinal leak and venous thromboembolism (VTE) are devastating complications of bariatric surgery. The performance of currently available predictive models for these complications remains wanting, while machine learning has shown promise to improve on traditional modeling approaches. The purpose of this study was to compare the ability of two machine learning strategies, artificial neural networks (ANNs), and gradient boosting machines (XGBs) to conventional models using logistic regression (LR) in predicting leak and VTE after bariatric surgery.
Methods
ANN, XGB, and LR prediction models for leak and VTE among adults undergoing initial elective weight loss surgery were trained and validated using preoperative data from 2015 to 2017 from Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program database. Data were randomly split into training, validation, and testing populations. Model performance was measured by the area under the receiver operating characteristic curve (AUC) on the testing data for each model.
Results
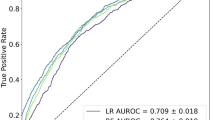
The study cohort contained 436,807 patients. The incidences of leak and VTE were 0.70% and 0.46%. ANN (AUC 0.75, 95% CI 0.73–0.78) was the best-performing model for predicting leak, followed by XGB (AUC 0.70, 95% CI 0.68–0.72) and then LR (AUC 0.63, 95% CI 0.61–0.65, p < 0.001 for all comparisons). In detecting VTE, ANN, and XGB, LR achieved similar AUCs of 0.65 (95% CI 0.63–0.68), 0.67 (95% CI 0.64–0.70), and 0.64 (95% CI 0.61–0.66), respectively; the performance difference between XGB and LR was statistically significant (p = 0.001).
Conclusions
ANN and XGB outperformed traditional LR in predicting leak. These results suggest that ML has the potential to improve risk stratification for bariatric surgery, especially as techniques to extract more granular data from medical records improve. Further studies investigating the merits of machine learning to improve patient selection and risk management in bariatric surgery are warranted.




Similar content being viewed by others
References
Nguyen NT, Varela JE (2017) Bariatric surgery for obesity and metabolic disorders: state of the art. Nat Rev Gastroenterol Hepatol 14:160
Böckelman C, Hahl T, Victorzon M (2017) Mortality following bariatric surgery compared to other common operations in Finland during a 5-year period (2009–2013). A nationwide registry study. Obes Surg 27:2444–2451. https://doi.org/10.1007/s11695-017-2664-z
Fry BT, Scally CP, Thumma JR, Dimick JB (2018) Quality improvement in bariatric surgery: the impact of reducing postoperative complications on medicare payments. Ann Surg 268:22–27
Funk LM, Jolles S, Fischer LE, Voils CI (2015) Patient and referring practitioner characteristics associated with the likelihood of undergoing bariatric surgery: a systematic review. JAMA Surg 150:999–1005
Funk LM, Jolles SA, Greenberg CC, Schwarze ML, Safdar N, McVay MA, Whittle JC, Maciejewski ML, Voils CI (2016) Primary care physician decision making regarding severe obesity treatment and bariatric surgery: a qualitative study. Surg Obes Relat Dis 12:893–901
Vidal J, Corcelles R, Jiménez A, Flores L, Lacy AM (2017) Metabolic and bariatric surgery for obesity. Gastroenterology 152:1780–1790
Alizadeh RF, Li S, Inaba C, Penalosa P, Hinojosa MW, Smith BR, Stamos MJ, Nguyen NT (2018) Risk factors for gastrointestinal leak after bariatric surgery: MBASQIP analysis. J Am Coll Surg 227:135–141. https://doi.org/10.1016/j.jamcollsurg.2018.03.030
Mocanu V, Dang J, Ladak F, Switzer N, Birch DW, Karmali S (2019) Predictors and outcomes of leak after Roux-en-Y Gastric Bypass: an analysis of the MBSAQIP data registry. Surg Obes Relat Dis 15:396–403
Turrentine FE, Denlinger CE, Simpson VB, Garwood RA, Guerlain S, Agrawal A, Friel CM, LaPar DJ, Stukenborg GJ, Jones RS (2015) Morbidity, mortality, cost, and survival estimates of gastrointestinal anastomotic leaks. J Am Coll Surg 220:195–206
Ward-Smith P (2012) Body mass index, surgery, and risk of venous thromboembolism in middle-aged women. Urol Nurs 32:220–223
Klovaite J, Benn M, Nordestgaard BG (2015) Obesity as a causal risk factor for deep venous thrombosis: a M endelian randomization study. J Intern Med 277:573–584
ASMBS Clinical Issues Committee (2013) ASMBS updated position statement on prophylactic measures to reduce the risk of venous thromboembolism in bariatric surgery patients. Surg Obes Relat Dis 9(4):493–497. https://doi.org/10.1016/j.soard.2013.03.006
Aminian A, Andalib A, Khorgami Z, Cetin D, Burguera B, Bartholomew J, Brethauer SA, Schauer PR (2017) Who should get extended thromboprophylaxis after bariatric surgery. Ann Surg 265:143–150
Dang JT, Switzer N, Delisle M, Laffin M, Gill R, Birch DW, Karmali S (2018) Predicting venous thromboembolism following laparoscopic bariatric surgery: development of the BariClot tool using the MBSAQIP database. Surg Endosc. https://doi.org/10.1007/s00464-018-6348-0
Gaborit B, Aron-Wisnewsky J, Salem J-E, Bege T, Frere C (2018) Pharmacologic venous thromboprophylaxis after bariatric surgery. Ann Surg 268:e51–e52
Thereaux J, Lesuffleur T, Czernichow S, Basdevant A, Msika S, Nocca D, Millat B, Fagot-Campagna A (2018) To what extent does posthospital discharge chemoprophylaxis prevent venous thromboembolism after bariatric Surgery? Results from a nationwide cohort of more than 110,000 patients. Ann Surg 267:727–733
Kumar SB, Hamilton BC, Wood SG, Rogers SJ, Carter JT, Lin MY (2018) Is laparoscopic sleeve gastrectomy safer than laparoscopic gastric bypass? A comparison of 30-day complications using the MBSAQIP data registry. Surg Obes Relat Dis 14:264–269. https://doi.org/10.1016/j.soard.2017.12.011
Bahl V, Hu HM, Henke PK, Wakefield TW, Campbell DA, Caprini JA (2010) A validation study of a retrospective venous thromboembolism risk scoring method. Ann Surg 251:344–350. https://doi.org/10.1097/SLA.0b013e3181b7fca6
Finks JF, English WJ, Carlin AM, Krause KR, Share DA, Banerjee M, Birkmeyer JD, Birkmeyer NJ, Collaborative MBS (2012) Predicting risk for venous thromboembolism with bariatric surgery: results from the Michigan Bariatric Surgery Collaborative. Ann Surg 255:1100–1104
Hashimoto DA, Rosman G, Rus D, Meireles OR (2018) Artificial intelligence in surgery: promises and perils. Ann Surg 268:70–76
Kim JS, Merrill RK, Arvind V, Kaji D, Pasik SD, Nwachukwu CC, Vargas L, Osman NS, Oermann EK, Caridi JM (2018) Examining the ability of artificial neural networks machine learning models to accurately predict complications following posterior lumbar spine fusion. Spine 43:853–860
Bertsimas D, Dunn J, Velmahos GC, Kaafarani HMA (2018) Surgical risk is not linear: derivation and validation of a novel, user-friendly, and machine-learning-based predictive optimal trees in emergency surgery risk (POTTER) Calculator. Ann Surg 268:574–583. https://doi.org/10.1097/SLA.0000000000002956
Goto T, Camargo CA, Faridi MK, Freishtat RJ, Hasegawa K (2019) Machine learning-based prediction of clinical outcomes for children during emergency department triage. JAMA Netw Open 2:e186937. https://doi.org/10.1001/jamanetworkopen.2018.6937
Wong A, Young AT, Liang AS, Gonzales R, Douglas VC, Hadley D (2018) Development and validation of an electronic health record–based machine learning model to estimate delirium risk in newly hospitalized patients without known cognitive impairment. JAMA Netw Open 1:e181018. https://doi.org/10.1001/jamanetworkopen.2018.1018
LeCun Y, Bengio Y, Hinton G (2015) Deep learning. Nature 521:436–444. https://doi.org/10.1038/nature14539
Chen T, Guestrin C (2016) Xgboost: a scalable tree boosting system. In: Proceedings of the 22nd acm sigkdd international conference on knowledge discovery and data mining. pp 785–794;
MBSAQIP. MBSAQIP participant use data file. https://www.facs.org/quality-programs/mbsaqip/participant-use. Accessed 14 Jan 2019
Pavlou M, Ambler G, Seaman SR, Guttmann O, Elliott P, King M, Omar RZ (2015) How to develop a more accurate risk prediction model when there are few events. BMJ 351:h3868
Lemaître G, Nogueira F, Aridas CK (2017) Imbalanced-learn: a python toolbox to tackle the curse of imbalanced datasets in machine learning. J Mach Learn Res 18:559–563
Millman KJ, Aivazis M (2011) Python for scientists and engineers. Comput Sci Eng 13:9–12
Oliphant TE (2007) Python for scientific computing. Comput Sci Eng 9:10–20
Anaconda Software Distribution. Computer software. Vers. 2-2.4.0. Anaconda, Nov. 2016. Web. https://anaconda.com.
McKinney W (2010) Data structures for statistical computing in python. In: Proceedings of the 9th Python in Science Conference, vol 445. pp 51–56
Oliphant TE (2006) A guide to NumPy. Trelgol Publishing USA
Collins GS, Reitsma JB, Altman DG, Moons KGM (2015) Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. Ann Intern Med 162:55. https://doi.org/10.7326/m14-0697
Paszke A, Gross S, Chintala S, Chanan G, Yang E, DeVito Z, Lin Z, Desmaison A, Antiga L, Lerer A (2017) Automatic differentiation in Pytorch.
Howard JAO (2018) Fastai. GitHub. https://github.com/fastai/fastai.
Seth Y (2018) A neural network in PyTorch for tabular data with categorical embeddings. Let the machines learn. https://yashuseth.blog/2018/07/22/pytorch-neural-network-for-tabular-data-with-categorical-embeddings/.
Ng A, Katanforoosh K. CS230 Deep learning course notes and code examples
Guo C, Berkhahn F (2016) Entity embeddings of categorical variables. arXiv preprint. arXiv: 160406737
Ioffe S, Szegedy C (2015) Batch normalization: accelerating deep network training by reducing internal covariate shift. arXiv
Caruana R, Lawrence S, Giles CL (2001) Overfitting in neural nets: backpropagation, conjugate gradient, and early stopping. Advances in neural information processing systems. MIT Press, Cambridge, pp 402–408
Srivastava N, Hinton G, Krizhevsky A, Sutskever I, Salakhutdinov R (2014) Dropout: a simple way to prevent neural networks from overfitting. J Mach Learn Res 15:1929–1958
Seabold S, Perktold J (2010) Statsmodels: Econometric and statistical modeling with python. In: Proceedings of the 9th Python in Science Conference, vol 57. p 61
DeLong ER, DeLong DM, Clarke-Pearson DL (1988) Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 44:837–845
Robin X, Turck N, Hainard A, Tiberti N, Lisacek F, Sanchez J-C, Müller M (2011) pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinf 12:77
Team RS (2015) RStudio: integrated development for R. RStudio Inc., Boston, p 42
Team RC (2013) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Wickham H (2016) ggplot2: elegant graphics for data analysis. Springer, New York
Pollard TJ, Johnson AEW, Raffa JD, Mark RG (2018) Tableone: an open source Python package for producing summary statistics for research papers. JAMIA Open 1:26–31. https://doi.org/10.1093/jamiaopen/ooy012
Frizzell JD, Liang L, Schulte PJ, Yancy CW, Heidenreich PA, Hernandez AF, Bhatt DL, Fonarow GC, Laskey WK (2017) Prediction of 30-day all-cause readmissions in patients hospitalized for heart failure: comparison of machine learning and other statistical approaches. JAMA Cardiol 2:204–209. https://doi.org/10.1001/jamacardio.2016.3956
Johnson KW, Soto JT, Glicksberg BS, Shameer K, Miotto R, Ali M, Ashley E, Dudley JT (2018) Artificial intelligence in cardiology. J Am Coll Cardiol 71:2668–2679
Golas SB, Shibahara T, Agboola S, Otaki H, Sato J, Nakae T, Hisamitsu T, Kojima G, Felsted J, Kakarmath S (2018) A machine learning model to predict the risk of 30-day readmissions in patients with heart failure: a retrospective analysis of electronic medical records data. BMC Med Inform Decis Mak 18:44
Telem DA, Yang J, Altieri M, Patterson W, Peoples B, Chen H, Talamini M, Pryor AD (2016) Rates and risk factors for unplanned emergency department utilization and hospital readmission following bariatric surgery. Ann Surg. 263:956–960. https://doi.org/10.1097/SLA.0000000000001536
Maier-Hein L, Vedula SS, Speidel S, Navab N, Kikinis R, Park A, Eisenmann M, Feussner H, Forestier G, Giannarou S (2017) Surgical data science for next-generation interventions. Nat Biomed Eng 1:691
Lee G, Rubinfeld I, Syed Z (2012) Adapting surgical models to individual hospitals using transfer learning. In: proceedings of the 2012 IEEE 12th International Conference on Data Mining Workshops, pp 57–63
Bihorac A, Ozrazgat-Baslanti T, Ebadi A, Motaei A, Madkour M, Pardalos PM, Lipori G, Hogan WR, Efron PA, Moore F (2019) MySurgeryRisk: development and validation of a machine-learning risk algorithm for major complications and death after surgery. Ann Surg 269:652–662
Natekin A, Knoll A (2013) Gradient boosting machines, a tutorial. Front Neurorobot 7:21
Masoomi H, Kim H, Reavis KM, Mills S, Stamos MJ, Nguyen NT (2011) Analysis of factors predictive of gastrointestinal tract leak in laparoscopic and open gastric bypass. Arch Surg 146:1048–1051. https://doi.org/10.1001/archsurg.2011.203
Qu Y, Cai H, Ren K, Zhang W, Yu Y, Wen Y, Wang J (2016) Product-based neural networks for user response prediction. In: Proceedings of the 2016 IEEE 16th International Conference on Data Mining. pp 1149–1154
Mikolov T, Sutskever I, Chen K, Corrado GS, Dean J (2013) Distributed representations of words and phrases and their compositionality. Advances in neural information processing systems. MIT Press, Cambridge, pp 3111–3119
Covington P, Adams J, Sargin E (2016) Deep neural networks for youtube recommendations. In: Proceedings of the 10th ACM conference on recommender systems. pp 191–198
Acknowledgements
The Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program (MBSAQIP) the hospitals participating in the MBSAQIP are the source of the data used herein; they have not verified and are not responsible for the statistical validity of the data analysis or the conclusions derived by the authors.
Funding
Supported by the Boston University Institute for Health System Innovation and Policy and the Boston Medical Center Department of Surgery. On a separate project, Dr. Bishara was supported by a National Institute of General Medical Sciences training grant (T32 GM008440, PI: Dexter Hadley).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Drs. Nudel and Bishara are co-founders of Bezel Health, a company building software to measure and improve healthcare quality interventions. Drs. Woodson, De Geus, Srinivasan, Patil, and Hess have no conflicts of interest or financial ties to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nudel, J., Bishara, A.M., de Geus, S.W.L. et al. Development and validation of machine learning models to predict gastrointestinal leak and venous thromboembolism after weight loss surgery: an analysis of the MBSAQIP database. Surg Endosc 35, 182–191 (2021). https://doi.org/10.1007/s00464-020-07378-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-020-07378-x