Abstract
Erythritol is a novelty 4-carbon sugar polyol and has great potential to be used as the precursor of some platform chemicals. The increasing cost of glucose poses researchers shifting insights to the cheaper biodiesel raw materials. Herein, we engineered a non-degradation, non-byproducts Yarrowia lipolytica for the erythritol production with high-titer from glycerol. Initially, the degradation and competition modules were blocked by URA3 counter-selection marker. Subsequently, a shortened biosynthetic pathway was explored to elevate its synthetic flux by multi-modules combination expression of functional genes. Furthermore, a screened glycerol transporter ScFPS1 was integrated into ERY6 genome to promote the glycerol uptake. The constructed strain ERY8 produced 176.66 g/L erythritol in the 5-L bioreactor with a yield and productivity of 0.631 g/g and 1.23 g/L/h, respectively, which achieved the highest fermentation production efficiency till date. This study proposed a novel multi-modules combination strategy for effectively engineering Y. lipolytica to produce erythritol using glycerol.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Erythritol (1,2,3,4-butanetetrol), known as a novelty 4-carbon sugar alcohol, is naturally abundant in fruits and vegetables, like pears, grapes or melons, where it exists as a metabolite or acts as an energy storage compound [1,2,3]. Due to its sweetness and low-calorie content combined with being non-cariogenic, erythritol mainly applied in the functional sugar field and is beneficial for diabetics and obesity. In addition, as the smallest molecular weight of all sugar alcohols, it also has potential applications in pharmaceutical, cosmetic and animal feed industries [2]. Moreover, erythritol serves as a promising platform chemical for synthesizing various valuable compounds, such as 1,4-anhydroerythritol, 1,3-butadiene, 1,4-butanediol, 2,5-dihydrofuran and tetrahydrofuran [4,5,6]. Therefore, the widespread application scenarios in various fields created a huge market for erythritol.
Due to the low efficiency of chemical synthesis and biological extraction, erythritol is mostly produced by microbial fermentation in the industrial production [7,8,9]. Currently, wild-type or mutant yeast strains that tolerate high osmotic pressure are primarily applied for the production of erythritol, including Aureobasidium [10], Candida [11], Pseudozyma [12], Torula [13], Trichosporon [14] and Yarrowia [9]. Among these yeasts, Yarrowia lipolytica is used as the excellent erythritol producer for its well-known genetic background and abundant genetic tools for modification [7, 15,16,17]. Most commonly, the main substrate consumed by the Y. lipolytica is glucose, which could obtain through enzymatic hydrolysis of corn or wheat starch. As demonstrated by Wang et al. (2020), the batch culture of the engineered Y. lipolytica HCY118 grown in a medium with an average glucose content of 300 g/L at 33 ℃, the final yield, production and productivity reached 0.65 g/g, 196 g/L and 2.51 g/L/h in the 30-m3 fermenter, respectively, showing great industrial potential of its industrialization [7]. However, considering the current unit cost of the main carbon source used in that process, the glucose-based erythritol production process may appear not economically feasible. Researchers are gradually expanding the carbon sources to the renewable feedstock and the main byproducts of biodiesel, such as crude or pure glycerol [8, 15].
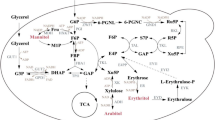
Yarrowia lipolytica is able to convert glycerol into different substances including erythritol. The pathway of erythritol synthesis from glycerol was elucidated clearly which involves a multi-module reaction (Fig. 1). The glycerol enters into cells with the assistance of glycerol transporters and is first phosphorylated by glycerol kinase (GUT1) and subsequently dehydrogenated by glycerol 3-phosphate dehydrogenase (GUT2) to produce dihydroxyacetone phosphate (DHAP). Then, isomerase (TPI1) isomerizes DHAP into glyceraldehyde 3-phosphate (G3P), which then enters into Embden-Meyerhof-Parnas pathway (EMP) and pentose phosphate pathway (PPP). The portion that flows into EMP providing raw materials, energy and cofactor for various life activities. The rest that flows into the PPP is converted into the key precursor erythrose 4-phosphate (E4P) under the catalysis of transketolase (TKL1) and transaldolase (TAL1), which can be further dephosphorylated and reduced by E4P phosphatase (E4PP) and erythrose reductase (ER) to produce erythritol. Besides, the catabolism of erythritol initiated by erythritol dehydrogenase (EYD1) is the main reason for its degradation during fermentation [18], while the byproducts represented by polyols [17] can compete with carbon flow to prevent efficient synthesis of erythritol and renders the downstream processing more challenging.
Metabolic strategies for enhancing erythritol production in Y. lipolytica. ArDH1 arabitol dehydrogenase, BP bisphosphate, CA citric acid, DHAP dihydroxyacetone phosphate, E4PP erythrose-4-phosphate phosphatase, ER erythrose reductase, EYD1 erythritol dehydrogenase, EYK1 erythrulose kinase, EYI1 erythrulose-1-phosphate isomerase, EYI2 erythrulose-4-phosphate isomerase, FBA1 fructose-bisphosphate aldolase, FBP1 fructose-1,6-bisphosphatase, GND1 phosphogluconate dehydrogenase, GUT1 glycerol kinase, GUT2 glycerol-3-phosphate dehydrogenase, HK hexokinase, MDH2 mannitol dehydrogenase, P phosphate, PFK1 phosphofructokinase, PGI1 glucose-6-phosphate isomerase, RKI1 ribulose-5-phosphate isomerase, RPE1 ribulose-5-phosphate 3-epimerase, SOL3 6-phosphogluconolactonase, TAL1 transaldolase, TCA tricarboxylic acid cycle TKL1 transketolase, TPI1 triosephosphate isomerase, ZWF1 glucose-6-phosphate dehydrogenase
Owing to its well characterized synthetic pathway, the breeding of industrial Y. lipolytica was gradually developed from early irrational mutagenesis, such as ultraviolet mutagenesis and ARTP mutagenesis, to rational systematic metabolic engineering. Major strategies for constructing Y. lipolytica producer of erythritol include strengthening the synthesis pathway, blocking the degradation pathway and enhancing the assimilation of glycerol. Mironczuk et al. (2016) first increased the glycerol uptake capacity in Y. lipolytica, resulting in a 35% increase in erythritol productivity by constitutive co-expression of GUT1 and GUT2 [19]. The key enzymes in the synthesis pathway were characterized and applied in metabolic modification to increase erythritol production [18, 20,21,22]. Zhang et al. (2021) achieved an erythritol production level of 52 g/L in 9 days by enhancing the precursors supply, blocking degradation pathway and redirecting the erythritol biosynthesis and the TCA cycle [16]. Jagtap et al. (2021) demonstrated the heterologous overexpression of sugar alcohol phosphatase (PYP) for increasing erythritol production in Y. lipolytica and further boosted the erythritol titer to 58.8 g/L in shake-flask experiments by combining the expression of native GUT1 and TKL1 [17]. Recently, Yang et al. (2022) reported a metabolically engineered Y. lipolytica strain that achieved a higher erythritol production level than ever reported previously [15]. Despite these successful examples of erythritol production using glycerol as the substrate, the efficient engineering of erythritol-producing cell factories for industrial-scale applications has been challenging. First, the single functional genes and their effective combination in the synthesis pathway showed differential ability in improving erythritol synthesis rate in different strains. Second, considerable amounts of byproducts, such as arabitol and mannitol, accumulated along with the production of erythritol, which could reduce the synthesis efficiency and yield. Additionally, the production of erythritol using glycerol as the substrate results in a long fermentation cycle and low productivity.
In this study, systems strategies were performed in Y. lipolytica A101 to obtain a highly efficient erythritol producer, which was non-degradation, non-byproducts, high-production and high-productivity by homologous recombination and random insertion. Firstly, we deleted the non-homologous end joint (NHEJ) regulator KU70 to improve its homologous recombination (HR) efficiency and further blocked the degradation pathway (non-degradation), eliminated the competition modules (non-byproducts) by the URA3 counter-selection. Subsequently, key genes for glycerol assimilation, reducing power and precursors supply were systematically screened and further combinatorial enhance the main biosynthesis pathway. Finally, the influx of glycerol was reinforced by transporter system modification. The resultant strain, ERY8, produced 177.66 g/L erythritol after 144 h of fermentation, with a productivity of 1.23 g/L/h and a yield of 0.631 g/g glycerol without byproducts.
Materials and methods
Strains, media and culture conditions
The Y. lipolytica strains and Escherichia coli used in this study are listed in Table 1. The E. coli strains were grown in Luria–Bertani (LB) medium (10 g/L tryptone, 5 g/L yeast extract and 10 g/L NaCl) supplemented with ampicillin (100 mg/L) or kanamycin (50 mg/L) at 37 ℃, 200 rpm/min. The Y. lipolytica strains were cultured at 30 ℃ in YPD medium (10 g/L yeast extract, 5 g/L tryptone and 10 g/L dextrose) for vegetative growth or in synthetic complete (SC) medium (6.7 g/L yeast nitrogen base without amino acids, 5 g/L ammonia sulfate and 20 g/L glucose) with appropriate nutrients (0.1 g/L uridine, 0.1 g/L L-leucine or/and 750 μg/mL hygromycin) for transformants screening at 30 ℃. In order to restore the vitality of frozen strains stored at – 80 ℃, the strains were first cultured overnight on a 30 ℃ YPD plate, and then, the single colonies were inoculated into a 24 well plate containing 3 mL YPD medium and cultured at 220 rpm.
Construction of plasmids
Primers used for plasmid construction are listed in Supplementary Table S1. To construct the KU70 deletion vector, the 1 kb upstream (up) and downstream (down) homologous sequences flanking the KU70 were amplified from Y. lipolytica A101 genome. The LEU2 selection marker was amplified from the pINA1269 plasmid. Then, the obtained up, down and LEU2 fragments were one-step fusion with pMD20 T-vector by Gibson assembly to obtain pdelku70. For the deletion of EYD1, MDH2 and ArDH1, we constructed a marker self-excision system. Briefly speaking, the 1 kb of upstream and downstream homologous sequences of the target gene were amplified from Y. lipolytica A101 genome. A downstream repeat sequence (Redown) about 500 bp was obtain by PCR using the 1 kb downstream sequence as the template. Subsequently, the URA3 marker, which was amplified from pINA1312 plasmid, was fusion with Up and Redown fragments by fusion-PCR. Finally, the fusion fragment was one-step assembled with the Down and pMD20 T-vector to obtain the corresponding deletion plasmids. For constructing the overexpression plasmids of key genes in the synthesis pathway, the PCR amplified GUT1, GUT2, TPI1, ZWF1, GND1, ER, TKL1, TAL1 fragments were separately cloned to the pINA1312 expression plasmid, which was linearized with BamHI. For the test of glycerol transporter performance, the putative glycerol transporters from Y. lipolytica or S. cerevisiae were PCR amplified from their genome and then cloned into the BamHI digested pINA1269 vector. The multi-genes combination expression plasmid was constructed by one-step fusion of the expression cassettes amplified from the single gene overexpression plasmids. For the effective glycerol transporter integration with the engineered strain ERY6, we fused the YlFPS2 and ScFPS1 fragments with the synthesis pUC19-hygR plasmid which was linearized with BamHI. All PCRs were conducted with the Phanta Max Super-Fidelity DNA Polymerase (Vazyme Biotech Co., Ltd, Nanjing, China). And the constructed plasmids are listed in Supplementary Table S2.
Y. lipolytica transformation and colony PCR
The linearized vectors with the deletion or expression cassettes were column purified using the TSP602-200 Trelief® DNA Gel extraction kit (TSINGKE, China). The transformation of Y. lipolytica with linearized vectors was performed using the lithium acetate method as previously described [17]. Transformation mixture was diluted and plated on SC agar plates with the corresponding nutrient and incubated at 30 ℃ for 2 ~ 3 days until colonies appeared. Yeast colonies were picked up and boiled in 10 μL 0.02 M NaOH for 10 min at 100 ℃. Primers and PrimeSTAR® HS PCR premix (TAKARA, Japan) were added to perform colony PCR. PCR products were Sanger sequenced to identify deletion or overexpression engineered strains.
Erythritol production in shake flasks
A single Y. lipolytica colony was picked from the plates to 50 mL seed medium (50 g/L glycerol, 3 g/L yeast extract, 3 g/L malt extract, 5 g/L bacteriological peptone) in 250-mL shake flasks. After being cultivated at 200 rpm and 30 ℃ for 24 h, it was transferred to 30 mL EPF medium. The composition of EPF medium was described by Zhang et al. (2020), which was made up of 100 g/L glycerol, 2.3 g/L (NH4)2SO4, 1 g/L MnSO4·7H2O, 0.23 g/L KH2PO4, 25 g/L NaCl, 1 g/L yeast extract and 3 g/L CaCO3 [16]. When necessary, 1 g/L uridine or/and 1 g/L L-leucine were added. The inoculated EPF mediums were cultivated at 30 ℃, 220 rpm for 6 days unless otherwise noted.
Erythritol production in 5-L bioreactor fermentation
The fed-batch fermentation was carried out for erythritol production in a 5-L bioreactor (Baoxing, Shanghai, China) with an effective working volume of 3-L at 30 ℃. The engineered Y. lipolytica strains were cultivated for seed preparation in 50-mL flask containing 5 mL of YPD medium. After overnight cultivation, the cells were inoculated in 250-mL flask containing 100 mL seed medium which contained 50.0 g/L glycerol, 3.0 g/L yeast extract, 3.0 g/L malt extract, 5.0 g/L peptone and incubated at 30 ℃, 220 rpm for 24 h. The seed culture was subsequently inoculated 0.3-L into 2.7-L fermentation seed medium in the 5-L bioreactor. The fermentation seed medium was made up of 130 g/L glycerol, 2.3 g/L (NH4)2SO4, 0.22 g/L KH2PO4, 1 g/L MgSO4·7H2O, 1 g/L yeast extract and 26.5 g/L NaCl. The dissolved oxygen was maintained at 20–40% by adjusting the stirrer speed and aeration rate. The temperature was maintained at 30 ℃, and the pH was automatically controlled at approximately 3.0 by adding 20% NaOH solution. The feeding medium which consists of 800 g/L glycerol, 4.6 g/L (NH4)2SO4, 0.22 g/L KH2PO4, 1 g/L MgSO4·7H2O, 1 g/L yeast extract and 21.3 g/L NaCl was added to the fermenter at 1.4 g/L/h constant feeding rate after 24 h until a final glycerol concentration of 280 g/L. The bioreactor with appropriate medium was sterilized in the autoclave at 121 ℃ for 20 min.
Determination of the fermentation parameters
The biomass or cell growth was measured by the optical absorbance at 600 nm (OD600) with the Eppendorf BioPhotometer D30 detector (Amersham Biosciences, Uppsala, Sweden). Erythritol, glycerol, mannitol and arabitol concentrations were determined by high-performance liquid chromatography (HPLC) equipped with a refractive index detector and an Aminex HPX-87H carbohydrate analysis column (Bio-Rad Laboratories, Inc., Hercules, CA). The column and guard cartridge were kept at 60 °C, and 5 mM H2SO4 was used as a mobile phase at a constant flow rate of 0.6 mL/min. All data represent means ± SD in this study.
Results and discussion
Construction of an initial chassis strain for erythritol accumulation
According to the previously reports, the non-homologous end-joint (NHEJ) is the dominant form of DNA repair in Y. lipolytica, which is mediated by the heterodimer protein complex Ku70/Ku80 [23]. The disruption of NHEJ system significantly increases the efficiency of HR so that facilitating the subsequent multiple genomic editing of Y. lipolytica [24]. Generally, either or both of the heterodimer Ku70/Ku80 was firstly chosen for disruption. Herein, we transformed the KU70 (YALI0C08701g) disruption cassette containing the left and right arms of the KU70 gene and the LEU2 marker into the host strain. Only one KU70 disrupted mutant was obtained by diagnostic PCR and sequencing from the selected 20 transformants, yielding the strain ΔKU70. Compared with the ΔLEU2 stain, ΔKU70 grew faster in the first four days and the yield of erythritol was the highest at the 144 h, reached to 35.48 g/L (a slightly higher than ΔLEU2), which may be caused by the LEU2 complementary (Fig. 2a). It was worth noting that the erythritol of both strains began to decline sharply from the 7th day (Fig. 2a), which mainly caused by the degradation of erythritol dehydrogenase gene (EYD1: YALI0F01650g) [18]. It has been reported that Y lipolytica was capable of synthesizing and utilizing erythritol [17, 18, 25]. Within the erythritol catabolic pathway, the EYD1 initially oxidized erythritol into erythrulose consuming one molecule of NADP+ and then phosphorylated into erythrulose-phosphate through an erythrulose kinase (EYK1: YALI0F01606g) [18, 25].
Comparison of fermentation performance between ERY1 and the initial strains. a The erythritol production and cell growth of the ΔLEU2 and ΔKU70 strains; b erythritol production and cell growth of ERY1 in the time-course fermentation; c the schematic diagram of the self-recombination between direct repeat (redown) of the EYD1 downstream sequence for marker curation; d counter-selection of URA3 under the pressure of 5-FOA and uridine
In order to test the effect of KU70 knockout on the efficiency of subsequent genetic engineering and avoid erythritol degradation, we purposefully selected EYD1 as the target gene for disruption. The transformants were identified by PCR and sequencing, and totally 13 EYD1 deletion strains (ERY1) were obtained from the 16 candidates. The HR efficiency greatly improved to 81.25%. The erythritol concentration of ERY1 was about 7.1% higher than that of the control on the 6th day (Fig. 2b), which reached 38.00 g/L, which further confirmed the EYD1 converts erythritol into erythrulose [18]. Besides, the detection of the byproducts during the fermentation process showed that after 144 h cultivation, the accumulation of arabitol reached 11.04 and 9.43 g/L, while mannitol reached 9.42 and 3.47 g/L, and the content of citric acid was 2.30 and 1.13 g/L in the ΔKU70 and ERY1 strains (Table 2), respectively. Among them, the byproducts of ERY1 in the 5 and 6 days showed a downward trend, presumably due to the cell's metabolism of other carbon sources caused by the blocking of erythritol consumption pathway.
In the genome editing of Y lipolytica, the curing or recycling of selection marker is a difficult problem that must be faced. Generally, the Cre/LoxP system was preferred in yeast, but its defect that every round of genome editing will introduce a lox site is obvious, probably leading to genomic instability after multiple rounds of gene modifications [26, 27]. In yeasts, the orotidine 5′-monophosphate decarboxylase (OMP) encoded by URA3 involved in uracil/uridine synthesis has been used as a sensitive and versatile auxotrophic marker. It can not only make up for the nutritional defect of URA3 deletion, but also make the strain be sensitive to 5-fluoroorotic acid (5-FOA). The deletion cassette of EYD1 was different from that of KU70 for an extra repeat sequence (redown) was inserted between the left arm and the URA3 fragment which could realize intramolecular HR under the selection condition so as to realize iterative metabolic engineering (Fig. 2c). The ERY1 strain was evenly plated on the SC solid medium containing 1 mg/L 5-FOA and 10 mM uridine (Fig. 2d). After being cultivated for 2 days, the URA3 marker self-excision mutants ERY1MSE were obtained (Fig. 1d). After deletion of KU70 and EYD1, we constructed the initial chassis for erythritol accumulation.
Eliminate the byproducts by blocking the competitive pathway
Some common intermediates, such as F-6-P, X-5-P, Ru-5-P and G-6-P, in the biosynthetic pathway of erythritol are also the precursors of byproducts [17]. The biosynthetic pathway of the byproducts competes with the carbon flux with erythritol, among which mannitol and arabitol account for the highest proportion, seriously increasing the difficulty and cost for the downstream separation and purification. Wang et al. systematically identified the biosynthetic genes that encoding enzymes with arabitol/mannitol dehydrogenase activities in Y. lipolytica [20]. Among all the characterized dehydrogenases, AraDH1 (YALI0F02211g) has the most robust activity towards mannitol, arabitol, xylitol and sorbitol, whereas MDH2 (YALI0D18964g) exhibited the strongest activity toward fructose.
To eliminate competing pathways and strengthen the carbon flux to erythritol, the gene ArDH1 (YALI0F02211g), MDH2 (YALI0D18964g), ArDH1 and MDH2 were deleted from the genome of the engineered strain ERY1, resulting strains ERY2-1, ERY2-2 and ERY3. The correctly integrated transformants were verified by diagnostic PCR with primers listed in Supplementary Table 1. The growth and production properties of the engineered strains ERY2-1, ERY2-2, ERY3 were tested in EPF medium with ERY1 as the control. The OD of the by-product eliminated strain was almost the same as that of the control strain in the fermentation medium (Fig. 3a), indicating that the byproducts do not have important physiological function in Y. lipolytica. ERY2-1 and ERY2-2 showed an improved erythritol synthesis ability to a certain extent (Fig. 3a). However, deletion of MDH2 and ArDH1 remarkedly accelerated the ability of chassis cells to synthesize erythritol. The titer of erythritol in ERY3 reached 45.59 g/L, about 19.97% higher than ERY1, after 144-h flask fermentation (Fig. 3b). Meanwhile, arabitol and mannitol could not be detected in the ERY3 (ΔMDH2ΔArDH1) strain during fermentation (Supplementary Fig. S1). We also found that the highest titer of citric acid increased to 4.24 g/L (Fig. 3c) and the OD600 reached 45.75 (Fig. 3b), indicating that elimination of both byproducts can enhance the central metabolic pathway and promote cell growth.
Effect of ArDH1 or/and MDH2 deletion on erythritol, byproducts production and cell growth. a The growth and production of ArDH1 or MDH2 knockout strains after six days of fermentation. ERY2-1: ERY1ΔArDH1; ERY2-2: ERY1ΔMDH2; b time-course analysis of fermentation performance of ERY3. ERY3:ERY1ΔArDH1ΔMDH2; c comparison of citric acid accumulation between ERY3 and ERY1 in the fermentation process
It was worth noting that deletion of MDH2 can completely eliminate the accumulation of mannitol, while ArDH1 knockout alone can only slightly reduce the production of arabitol (Fig. 3a). In Y. lipolytica, MDH2 was proved to be the key gene of biosynthesis of mannitol, which is also the theoretical basis for us to knock it out. Because of the redundancy of dehydrogenase function [7], the arabinol synthesis gene has not been fully clarified. We tried to disrupt ArDH1 in order to reduce the accumulation of arabitol to some extent, but its deletion did not achieve the desired purpose. Unexpectedly, deletion of ArDH1 and MDH2 can effectively eliminate the accumulation of byproducts (Supplementary Fig. S1). We deduced that it is the redundancy of dehydrogenase function that leads to the above results; that is, both ArDH1 and MDH2 enzymes have the ability to catalyze d-Xylulose to synthesize arabitol [7]. Overall, our results demonstrated that the competing pathways of erythritol were successfully blocked and further improve the titer of erythritol.
Effective multi-genes co-expression strategy to enhance erythritol biosynthesis
Most of the genes involved in the erythritol biosynthesis from glycerol in Y. lipolytica have been functionally identified (Fig. 1) [16, 17, 20, 21, 28]. They were divided into 3 functional modules, namely glycerol utilization (GUT1, GUT2 and TPI1), reducing power cycle (ZWF1, GND1, ER) and precursor supply (TKL1, TAL1, RKI1, RPE1). In order to determine the optimal combination of genes for enhancing erythritol synthesis, we systematically evaluated the performance of the key genes in modules. In the glycerol uptake module, we assessed the effect of GUT1, GUT2 and TPI1 overexpression on the erythritol production and glycerol absorption. After 4-day flask fermentation, the residual glycerol of ERY4-1, ERY4-2 and ERY4-3 was slightly higher than the control strain, but the engineered strains showed a higher cell density (Fig. 4a), which suggested that glycerol assimilation modules are beneficial for cell growth. For strain ERY4-1 (pINA1312-GUT1), the erythritol titer, OD600 increased to 25.60 g/L and 35.81, which were increased by 30.15 and 18.50% compared to the ΔKU70 strain, respectively (Fig. 4a). The overexpression of GUT2 (ERY4-2) and TPI1 (ERY4-3) could not improve the erythritol titer (Fig. 4a). Hence, our results further confirmed GUT1 as the most important gene in the glycerol module, which was consistent with the previous results [15, 16].
Overexpression of key genes involved in the synthesis of erythritol from glycerol individually or combination in different engineered strains. Production of erythritol and growth of engineered strains harboring the tested genes in a glycerol assimilation module. ERY4-1: hp4d-GUT1; ERY4-2: hp4d-GUT2; ERY4-3: hp4d-TPI1; b reducing power module. ERY4-4: hp4d-ZWF1; ERY4-5: hp4d-GND1; ERY4-6: hp4d-ER; c precursors supply module. ERY4-7: hp4d-TAL1; ERY4-8: hp4d-TKL1; d multi-modules combination. ERY5-1: Php4d-TKL1-TAL1; ERY5-2: Php4d-TKL1-TAL1-ER; ERY5-3: Php4d-TKL1-TAL1-ER-GUT1. e Analysis of erythritol production performance by strain ERY6 driven by the effective multi-modules combination in flask fermentation. ERY6: ERY3MSE, pINA1312-OEGUT1-ER-TKL1-TAL1
The supply of NADPH is presumed as an important factor in erythritol production since the reactions catalyzed by erythrose reductase (ER) are NADPH dependent, consuming 1 molecules of NADPH in the biosynthesis of 1 molecule of erythritol in Y. lipolytica [20]. Meanwhile, the glucose-6P dehydrogenase ZWF1 (YALI0E22649g) and 6-phosphogluconate dehydrogenase GND1 (YALI0B15598g) of the PPP oxidative phase are the key genes for generating NADPH [7, 16]. Previous researches suggested the ZWF1 and GND1 have a major role in NADPH supplying for ER [16]. We further constructed ZWF1, GND1 and ER overexpression strain ERY4-4, ERY4-5 and ERY4-6, respectively. Although the growth of strain ERY4-6 was lower than that of ΔKU70, ERY4-4 and ERY4-5, the final concentration of erythritol significantly increased to 25.28 g/L (about 30% higher than the control strain) after 4 days cultivation (Fig. 4b), which was caused by the strong metabolic pull force of ER [16, 21, 28]. Unexpectedly, overexpression of ZWF1 and GND1 had less effect on the increase of erythritol production, and their strengthening leads to a decline in cell growth (Fig. 4b). Our results were inconsistent with that of reported by Wang et al. (2018), who co-overexpressed the ZWF1 and GND1 in the engineered strain yielding an increase of 6% erythritol titer [20]. Meanwhile, the co-overexpression of ZWF1, GND1 and ER27 in the MY10 strain had no effect on improving the performance of the strain [16]. These inconsistent results may be caused by the differential initial production performance of the target strains and differential in gene expression level. For the model strain, due to the low initial titer of erythritol, the modification of a single gene or multi-genes can significantly show their impact on the strain performance [16, 28]. But for the industrial strain, such as CGMCC7326, its initial erythritol titer reached 152 ± 4 g/L [20], which means that there is limited space for metabolic modification to enhance the production capacity of strains. Besides, the factors, such as copy number, promoter and the insertion site in the genome, can affect the target gene expression strength. Zhang et al. (2020) enhanced the expression of GND1, ZWF1 and ER by CRISPR/Cas9 mediated single copy integration [16], while Wang et al. (2018) overexpressed GND1 and ZWF1 by 26S rDNA mediated multi-copies integration [20]. In our study, the zeta sequences mediated multi-copies integration was used to overexpress GND1 and ZWF1. Although these works did not quantify the inserted copies by qPCR, there is a high probability that the integration number of these genes differs, because the 26 s rDNA and zeta sequences exist in the multi-copies form in the genome. In addition, we deduced that the NADPH consumed by ER may come from other metabolic processes of cells, which needs further verification. It was worth noting that the decreased cell growth of ERY4-6 may be caused by the enhanced ER activity, which pulled the PPP product E-4-P transformed into erythritol rapidly, thus indirectly improving the strength of the PPP and competing with EMP and TCA for carbon flow. GND1 and ZWF1 are key genes in the PPP oxidative phase, competing with the EMP for G-6-P, directly leading to a decrease in EMP metabolism.
On the other hand, we suggested that the synthesis of erythritol from glycerol may cause waste of carbon flow through the PPP oxidation phase, because the intermediates F-6-P and GA-3-P can be one-step catalyzed by TKL1 to obtain X-5-P and E-4-P, which avoided at least 4 steps in the PPP oxidation phase (Fig. 1). This hypothesis was further confirmed by enhancing TKL1 and TAL1 of the precursor supply module in the ΔKU70 strain. Compared with ΔKU70 strain, the titer of erythritol in ERY4-7 (pINA1312-TAL1) and ERY4-8 (pINA1312-TKL1) increased by 26.39% and 30.66%, reaching 24.86 g/L and 25.70 g/L, respectively (Fig. 4c). It was reported that overexpression of transketolase (TKL1) and transaldolase (TAL1) genes can significantly increase erythritol production and reduce fermentation time by effectively precursor supply [15,16,17, 22, 28]. We further co-expressed GUT1, ER, TKL1 and TAL1 in ERY1MSE to obtained strain ERY5-1 (Php4d-TKL1-TAL1), ERY5-2 (Php4d-TKL1-TAL1-ER) and ERY5-3 (Php4d-TKL1-TAL1-ER-GUT1). Multi-genes combination expression mainly enhanced the PPP pathway and competed with TCA and EMP for carbon flux. Thus, these engineered strains showed slower growth rate, but higher erythritol titer. Especially for strain ERY5-3, it could produce 54.02 g/L erythritol after 6 days flask fermentation achieving YERY 0.54 g/g, QERY 0.375 g/L/h (Fig. 4d). It should be pointed out that the growth of ERY5-3 was partially restored compared with ERY5-1 and ERY5-2, which may be caused by the growth promotion effect of GUT1. Subsequently, we introduced the optimal combination pINA1312-GUT1-TKL1-TAL1-ER into the byproducts eliminated strain ERY3MSE to obtain ERY6. According to the time-course fermentation parameters of 6 days (Fig. 4e), the glycerol uptake rate of ERY6 was slow in the first three days. From 72 h, with the rapid synthesis of erythritol, the consumption rate of glycerol also increased significantly. The production of erythritol continued to accumulate until the 6th day of fermentation, which demonstrated that the simultaneous overexpression of the rate-limiting genes in ERY3 significantly enhanced erythritol production, producing 62.58 g/L erythritol with a yield of 0.626 g/g and QERY 0.435 g/L/h. These results suggested the introduction of the multi-genes co-expression successfully diverted more carbon flow from F-5-P and GA-3-P to the synthesis of erythritol instead of flowing to the TCA cycle.
Modification of the glycerol transport system
In the industrial production of erythritol, chassis cells need to tolerate high concentration of initial carbon source. In addition, the production cycle with glycerol as the carbon source is longer (more than seven days), which greatly increases the production cost. In the utilization process of hexose and pentose pathway, the excavation and transformation of its transport protein can greatly improve the utilization of corresponding carbon sources [29, 30], which provides ideas for the utilization of glycerol. Previously, Anna et al. identified six transporters involved in low concentration (< 25 g/L) glycerol uptake and experimentally confirmed to have glycerol transport activity in Y. lipolytica [31]. However, the deletion of identified glycerol transporter has little effect on cell growth at high glycerol concentrations (100 g/L). Hence, researchers mentioned that passive diffusion is the main uptake mode of glycerol at high concentration, which was subsequently corrected to FPS1 or other less specific proteins facilitating the passive diffusion of glycerol [32].
To further improve the production of erythritol and glycerol consumption rate, it is of great interest to explored which of the putative glycerol transporters are essential for the import of glycerol into the Y. lipolytica cells at high concentration. The glycerol transporter overexpression strains (YlFPS1, YlFPS2, YlSTL1, YlSTL2, YlSTL3, YlSTL4, YlSTL5, YlSTL6, YlSTL7, YlSTL8, ScFPS1 and ScSTL1) were obtained by diagnostic PCR and sequencing. Subsequently, we tested the growth and production characteristics of all engineering strains during the shaking flask fermentation process of 120 h. Compared with the wild type Y. lipolytica, increase of YlFPS1, YlFPS2, YlSTL2, ScSTL1 and ScFPS1 copy number did significantly promote the glycerol consumptions and erythritol production at 100 g/L glycerol (Fig. 5a and c). As a member of the major facilitator superfamily (MFS), STL1 from S. cerevisiae was prove to be an active symporter of glycerol and H+ [33]. But most STL1 homologs from Y. lipolytica had no positive effect on glycerol uptake and conversion under the condition of high concentration of glycerol, except for YlSTL2. YlSTL2 shares 86% homology with STL1 and its overexpression did not improve cell growth, but its glycerol uptake rate is the fastest (Fig. 5a and b). After fermentation for 120 h, YlSTL2 accumulated 1.87-fold arabitol, 1.65-fold mannitol and 2.15-fold citric acid compared with WT (Supplementary Fig. S2), which was the reason for the decline in erythritol production after 96 h of fermentation (Fig. 5c).
Screening of glycerol transporters and their application in engineering strains. a Residual glycerol content; b the biomass and c the erythritol titer of strains overexpressing glycerol transporters at a concentration of 100 g/L glycerol; d residual glycerol content; e the biomass and f the erythritol titer of engineered strains ERY7 and ERY8 at 100 g/L glycerol cultured for 5 days. ERY6 served as the control
It is interesting to note that the overexpression of the major intrinsic protein (MIPs) YlFPS2 could elevate the OD600 value to 38.08 after 5-day cultivation, a 18.26% higher than that of the control strain (Fig. 5b). Meanwhile, the overexpression of FPS2 led to 32.49% increase in erythritol production (Fig. 5c). Besides, we found that the enhancement of homologous FPS1 (YlFPS1) significantly inhibited the growth of the strain (Fig. 5b), while promoted the erythritol titer to 35.21 g/L (increased by 27.80%) (Fig. 5c). It was reported that when the strains cultivated in high glycerol concentration, the two-directional transporter YlFPS1 mainly played an efflux role [31]. The heterologous ScFPS1 significantly increased erythritol production with little impact on cell growth (Fig. 5b and c). Therefore, YlFPS2 and ScFPS1 under the hp4d promoter were further separately inserted into the genome of engineering strain ERY6 strain, generating strain ERY7 and ERY8. Compared with ERY6, ERY8 showed a stronger glycerol utilization ability, and the glycerol in the culture medium has been exhausted at 120 h (Fig. 5d). Although ERY6 does not enhance the glycerol transport system, its growth is almost consistent with ERY7 and ERY8 (Fig. 5e), possibly due to the strong growth promoting ability of GUT1. The erythritol titer of ERY7 (Php4d-YlFPS2) and ERY8 (Php4d-ScFPS1), respectively, increased to 62.12 g/L and 64.65 g/L, with the yield reaching 0.621 g/g and 0.647 g/g glycerol in the flask fermentation and the fermentation cycle was shortened to 120 h. In addition, ERY8 produced 1.19 g/L citric acid at 120 h, only 30.93% of ERY6 (Supplementary Fig. S2), suggesting that the carbon flux flowing to the TCA cycle decreased. These results proved that ScFPS1 could efficiently facilitate the strain consume the substrates under the condition of high concentration glycerol, promoting glycerol assimilation process, thus improving the erythritol titer.
Fed-Batch fermentation for erythritol in a 5-L fermenter
To further evaluate the characteristics of ERY3, ERY6 and ERY8, fed-batch fermentation was conducted in a 5-L bioreactor, using ΔKU70 strain as a control. Cell growth, erythritol and byproducts production during fermentation are shown in Fig. 6. The cell growth of all the strains steadily increased and the biomass entered into the platform stage after 72-h cultivation. However, the biomass (OD600) of ERY3 reached a maximum of 112.33, with about 13.92% higher than that of the ΔKU70 (98.60). Accumulation of erythritol was detected every 24 h and the titer reached a peak of 137.97 g/L with a productivity of 0.96 g/L/h and a yield of 0.49 g/g glycerol after 144 h of cultivation, which was 43.02% higher than that of the ΔKU70 (96.47 g/L). These results demonstrated that eliminating competitive pathways can promote the erythritol production, but also strengthen the TCA cycle and promote cell growth, resulting in a waste of carbon flow (Fig. 6b). As for the strain EYR6, while the maximum OD600 was significantly less than that of ΔKU70, the cell performance was much better than that of ΔKU70 after the strains quickly entered the plateau growth phase. The erythritol titer, yield and productivity of strain ERY6 reached 167.82 g/L, 0.60 g/g glycerol and 1.17 g/L/h, respectively, which were significantly higher than those of ERY3 and ΔKU70. The above results further proved that enhancing erythritol precursors promotes its production, but reducing cell biomass. Furthermore, the growth of strain ERY8 modified by the glycerol transport system was almost identical to that of strain ERY6. In the same period of time, the titer of ERY8 was accordingly promoted, achieving 176.66 g/L of erythritol, which were 28.04% and 5.27% higher than those of ERY3 and ERY6, respectively. The yield reached 0.631 g/g glycerol, improving 37.17% and 5.2%, respectively, compared with those of ERY3 and ERY6. And its productivity was 1.23 g/L/h. It is noteworthy that the yield and productivity of strain ERY8 has reached 159.39 g/L and 1.33 g/L/h at the 5th day, indicating that the glycerol transporter ScFPS1 effectively promotes glycerol uptake and improves the production in the tank. As expected, the byproducts, such as arabitol and mannitol, were undetectable throughout the fermentation process (data not shown).
Currently, many studies have been carried out to produce erythritol by fermentation with pure glycerol as substrate [16,17,18, 22, 28]. These works mainly focused on mining and evaluating the impact of key genes in erythritol synthesis pathway, and the positive factors were not efficiently combined, resulting in a lower yield and productivity than ERY8. The engineered strain ERY8 determined the optimal combination of synthesis pathway genes, while also exploring a new transporter protein (ScFPS1) to promote the utilization of glycerol, improving the conversion rate and spatiotemporal yield. The erythritol yield and productivity of the engineered strain (OEGUT1-GUT2-TKL1, ΔEYD1) that fermented with crude glycerol in 5-L bioreactor reached 150 g/L and 1.25 g/L/h, respectively [15], but its performance was still lower than ERY8, suggesting that the elimination of byproducts and the enhancement of glycerol transport contributed to the further improvement of strain production performance. During the scale-up of erythritol production from glycerol using the Y. lipolytica MK1, the conversion rate was 0.533 g/g, which was lower than ERY8, although its yield and productivity reached 180.3 g/L and 1.25 g/L/h [8]. In general, the modification of ERY8 provided a new idea for improving the performance of erythritol producing strains.
Conclusion
In this study, a Y. lipolytica chassis for producing erythritol was constructed by blocking the degradation and competition pathway, enhancing the carbon flux to erythritol synthesis and boosting the glycerol influx. The final strain engineered here, ERY8, achieved the greatest erythritol production, yield and productivity, with a shortened fermentation time. Moreover, no detectable byproducts had accumulated during fermentation. Not only does ERY8 present the highest erythritol production performance, but it also paves the way for the industrial production of erythritol using the inexpensive biomass raw material glycerol by Y. lipolytica.
References
Carly F, Fickers P (2018) Erythritol production by yeasts: a snapshot of current knowledge. Yeast 35:455–463
Regnat K, Mach RL, Mach-Aigner AR (2018) Erythritol as sweetener-wherefrom and whereto? Appl Microbiol Biotechnol 102:587–595
Moon HJ, Jeya M, Kim IW, Lee JK (2010) Biotechnological production of erythritol and its applications. Appl Microbiol Biotechnol 86:1017–1025
Nakagawa Y, Kasumi T, Ogihara J, Tamura M, Arai T, Tomishige K (2020) Erythritol: another C4 platform chemical in biomass refinery. ACS Omega 5:2520–2530
Sivaraman B, Badle SS, Rangaswamy V (2016) Production of 1,3-butadiene from erythritol. ChemistrySelect 1:4630–4632
Dai L, Tai C, Shen YL, Guo YL, Tao F (2019) Biosynthesis of 1,4-butanediol from erythritol using whole-cell catalysis. Biocatal Biotransfor 37:92–96
Wang N, Chi P, Zou Y, Xu Y, Xu S, Bilal M, Fickers P, Cheng H (2020) Metabolic engineering of Yarrowia lipolytica for thermoresistance and enhanced erythritol productivity. Biotechnol Biofuels 13:176
Rakicka-Pustulka M, Mironczuk AM, Celinska E, Bialas W, Rymowicz W (2020) Scale-up of the erythritol production technology - Process simulation and techno-economic analysis. J Clean Prod 257:120533
Mironczuk AM, Rakicka M, Biegalska A, Rymowicz W, Dobrowolski A (2015) A two-stage fermentation process of erythritol production by yeast Y. lipolytica from molasses and glycerol. Bioresource Technol 198:445–455
Guo J, Li JX, Chen YF, Guo XW, Xiao DG (2016) Improving erythritol production of Aureobasidium pullulans from xylose by mutagenesis and medium optimization. Appl Biochem Biotech 180:717–727
Kohl ES, Leet TH, Lee DY, Kim HJ, Ryu YW, Seo JH (2003) Scale-up of erythritol production by an osmophilic mutant of Candida magnoliae. Biotechnol Lett 25:2103–2105
Jeya M, Lee KM, Tiwari MK, Kim JS, Gunasekaran P, Kim SY, Kim IW, Lee JK (2009) Isolation of a novel high erythritol-producing Pseudozyma tsukubaensis and scale-up of erythritol fermentation to industrial level. Appl Microbiol Biot 83:225–231
Lee JK, Koo BS, Kim SY (2002) Fumarate-mediated inhibition of erythrose reductase, a key enzyme for erythritol production by Torula corallina. Appl Environ Microbiol 68:4534–4538
Sawada K, Taki A, Yamakawa T, Seki M (2009) Key role for transketolase activity in erythritol production by Trichosporonoides megachiliensis SN-G42. J Biosci Bioeng 108:385–390
Yang S, Pan X, Wang Q, Lv Q, Zhang X, Zhang R, Rao Z (2022) Enhancing erythritol production from crude glycerol in a wild-type Yarrowia lipolytica by metabolic engineering. Front Microbiol 13:1054243
Zhang L, Nie MY, Liu F, Chen J, Wei LJ, Hua Q (2021) Multiple gene integration to promote erythritol production on glycerol in Yarrowia lipolytica. Biotechnol Lett 43:1277–1287
Jagtap SS, Bedekar AA, Singh V, Jin YS, Rao CV (2021) Metabolic engineering of the oleaginous yeast Yarrowia lipolytica PO1f for production of erythritol from glycerol. Biotechnol Biofuels 14:188
Carly F, Steels S, Telek S, Vandermies M, Nicaud JM, Fickers P (2018) Identification and characterization of EYD1, encoding an erythritol dehydrogenase in Yarrowia lipolytica and its application to bioconvert erythritol into erythrulose. Bioresour Technol 247:963–969
Mironczuk AM, Rzechonek DA, Biegalska A, Rakicka M, Dobrowolski A (2016) A novel strain of Yarrowia lipolytica as a platform for value-added product synthesis from glycerol. Biotechnol Biof. https://doi.org/10.1186/s13068-016-0593-z
Cheng H, Wang S, Bilal M, Ge X, Zhang C, Fickers P, Cheng H (2018) Identification, characterization of two NADPH-dependent erythrose reductases in the yeast Yarrowia lipolytica and improvement of erythritol productivity using metabolic engineering. Microb Cell Fact 17:133
Janek T, Dobrowolski A, Biegalska A, Mironczuk AM (2017) Characterization of erythrose reductase from Yarrowia lipolytica and its influence on erythritol synthesis. Microb Cell Fact 16:118
Carly F, Vandermies M, Telek S, Steels S, Thomas S, Nicaud JM, Fickers P (2017) Enhancing erythritol productivity in Yarrowia lipolytica using metabolic engineering. Metab Eng 42:19–24
Abdel-Mawgoud AM, Stephanopoulos G (2020) Improving CRISPR/Cas9-mediated genome editing efficiency in Yarrowia lipolytica using direct tRNA-sgRNA fusions. Metab Eng 62:106–115
Verbeke J, Beopoulos A, Nicaud JM (2013) Efficient homologous recombination with short length flanking fragments in Ku70 deficient Yarrowia lipolytica strains. Biotechnol Lett 35:571–576
Niang PM, Arguelles-Arias A, Steels S, Denies O, Nicaud JM, Fickers P (2020) In Yarrowia lipolytica erythritol catabolism ends with erythrose phosphate. Cell Biol Int 44:651–660
Solis-Escalante D, van den Broek M, Kuijpers NG, Pronk JT, Boles E, Daran JM, Daran-Lapujade P (2015) The genome sequence of the popular hexose-transport-deficient Saccharomyces cerevisiae strain EBYVW4000 reveals LoxP/Cre-induced translocations and gene loss. FEMS Yeast Res 15:332
Darvishi F, Ariana M, Marella ER, Borodina I (2018) Advances in synthetic biology of oleaginous yeast Yarrowia lipolytica for producing non-native chemicals. Appl Microbiol Biotechnol 102:5925–5938
Mironczuk AM, Biegalska A, Dobrowolski A (2017) Functional overexpression of genes involved in erythritol synthesis in the yeast Yarrowia lipolytica. Biotechnol Biofuels 10:77
Lazar Z, Neuveglise C, Rossignol T, Devillers H, Morin N, Robak M, Nicaud JM, Crutz-Le Coq AM (2017) Characterization of hexose transporters in Yarrowia lipolytica reveals new groups of Sugar Porters involved in yeast growth. Fungal Genet Biol 100:1–12
Wei W, Zhang P, Shang Y, Zhou Y, Ye BC (2020) Metabolically engineering of Yarrowia lipolytica for the biosynthesis of naringenin from a mixture of glucose and xylose. Bioresour Technol 314:123726
Erian AM, Egermeier M, Marx H, Sauer M (2022) Insights into the glycerol transport of Yarrowia lipolytica. Yeast 39:323–336
Oliveira R, Lages F, Silva-Graca M, Lucas C (2003) Fps1p channel is the mediator of the major part of glycerol passive diffusion in Saccharomyces cerevisiae: artefacts and re-definitions. Yeast 20:S249–S249
Ferreira C, van Voorst F, Martins A, Neves L, Oliveira R, Kielland-Brandt MC, Lucas C, Brandt A (2005) A member of the sugar transporter family, Stl1p is the glycerol/H+ symporter in Saccharomyces cerevisiae. Mol Biol Cell 16:2068–2076
Acknowledgements
This research was financially supported by the Key R&D Program of Zhejiang (2023C01113).
Author information
Authors and Affiliations
Contributions
L-gH contributed to the conceptualization, methodology, writing—review and editing. B-wX was involved in writing—original draft, methodology and validation. W-jW and LN were involved in the plasmid construction and validation. H-y Wang assisted in the methodology and validation. WY performed the methodology and validation. J-pZ and BZ contributed to the investigation; Z-QL and Y-GZ contributed to the project administration, funding acquisition, supervision and conceptualization.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, Lg., Xiao, Bw., Wang, Wj. et al. Multiplex modification of Yarrowia lipolytica for enhanced erythritol biosynthesis from glycerol through modularized metabolic engineering. Bioprocess Biosyst Eng 46, 1351–1363 (2023). https://doi.org/10.1007/s00449-023-02906-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-023-02906-0