Abstract
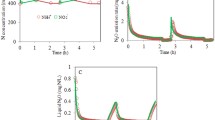
A comprehensive model for nitrous oxide (N2O) emissions in an anaerobic/oxygen-limited aerobic (A/OLA) process is proposed here. This paper includes the following main innovations: (i) adding the phosphorus-accumulating organism (XPAO) denitrification pathway to the contribution of N2O emissions; (ii) considering the biological removal of organic matter and phosphorus and predicting the effect of influent phosphorus concentration on N2O emissions via an increase in the influent phosphorus concentration; and (iii) determining the effect of XPAO on N2O production in a simultaneous nitrification, denitrification and phosphorus removal (SNDPR) system by sensitivity analysis. The results suggested that the simulated data matched the measured data well. The predominant pathways of N2O emissions in the process of A/OLA were the ammonium-oxidizing bacterium (XAOB) denitrification pathway and the heterotrophic bacterium (XH) denitrification pathway, while the incomplete hydroxylamine (NH2OH) oxidation pathway and the XPAO denitrification pathway contributed less to N2O emissions. The metabolic activity of XPAO had a significant effect on N2O emissions, and increasing the influent phosphorus concentration was beneficial for reducing the release of N2O. This study is expected to provide a meaningful reference for reducing N2O emissions in wastewater treatment engineering.






Similar content being viewed by others
References
Ciais P, Sabine C, Bala G, Bopp L, Brovkin V, Canadell A, Chhabra R, DeFries R, Galloway J, Heimann C, Jones C, Le Quéré C, Myneni RB, Piao S, Thornton P (2009) Carbon and other biogeochemical cycles BT: climate change the physical science basis. contribution of working group I to the fifth assessment report of the intergovernmental panel on climate change. Cambridge University Press, Cambridge
Ravishankara AR, Daniel JS, Portmann RW (2009) Nitrous oxide (N2O): the dominant ozone-depleting substance emitted in the 21st century. Science 326(5949):123–125
Division ECC (2018) Inventory of U.S. Greenhouse Gas Emissions and Sinks: 1990–2016. United States Environmental protection Agency
Samie G, Bernier J, Rocher V, Lessard P (2011) Modelling nitrogen removal for a denitrification biofilter. Bioprocess Biosyst Eng 34(6):747–755. https://doi.org/10.1007/s00449-011-0524-0
Tallec G, Garnier J, Gousailles M (2006) Nitrogen removal in a wastewater treatment plant through biofilters: nitrous oxide emissions during nitrification and denitrification. Bioprocess Biosyst Eng 29(5–6):323–333. https://doi.org/10.1007/s00449-006-0081-0
Ni BJ, Pan Y, van den Akker B, Ye L, Yuan Z (2015) Full-scale modelling explaining large spatial variations of nitrous oxide fluxes in a step-feed plug-flow wastewater treatment reactor. Environ Sci Technol 49(15):9176–9184. https://doi.org/10.1021/acs.est.5b02038
Hiatt WC, Grady CP Jr (2008) An updated process model for carbon oxidation, nitrification, and denitrification. Water Environ Res 80(11):2145–2156. https://doi.org/10.2175/106143008x304776
Guo L, Vanrolleghem PA (2014) Calibration and validation of an activated sludge model for greenhouse gases no. 1 (ASMG1): prediction of temperature-dependent N(2)O emission dynamics. Bioprocess Biosyst Eng 37(2):151–163
Sperandio M, Pocquet M, Guo L, Ni BJ, Vanrolleghem PA, Yuan Z (2016) Evaluation of different nitrous oxide production models with four continuous long-term wastewater treatment process data series. Bioprocess Biosyst Eng 39(3):493–510. https://doi.org/10.1007/s00449-015-1532-2
Pocquet M, Wu Z, Queinnec I, Sperandio M (2016) A two pathway model for N2O emissions by ammonium oxidizing bacteria supported by the NO/N2O variation. Water Res 88:948–959. https://doi.org/10.1016/j.watres.2015.11.029
Ding X, Zhao J, Hu B, Li X, Ge G, Gao K, Chen Y (2017) Mathematical modelling of nitrous oxide (N2O) production in anaerobic/anoxic/oxic processes: improvements to published N2O models. Chem Eng J 325:386–395. https://doi.org/10.1016/j.cej.2017.05.082
Ni BJ, Ruscalleda M, Pellicer-Nacher C, Smets BF (2011) Modelling nitrous oxide production during biological nitrogen removal via nitrification and denitrification: extensions to the general ASM models. Environ Sci Technol 45(18):7768–7776. https://doi.org/10.1021/es201489n
Domingo-Félez C, Smets BF (2020) Modelling N2O dynamics of activated sludge biomass: uncertainty analysis and pathway contributions. Chem Eng J. https://doi.org/10.1016/j.cej.2019.122311
Law Y, Lant P, Yuan Z (2013) The confounding effect of nitrite on N2O production by an enriched ammonia-oxidizing culture. Environ Sci Technol 47(13):7186–7194. https://doi.org/10.1021/es4009689
Mannina G, Cosenza A, Ekama GA (2017) Greenhouse gases from membrane bioreactors: mathematical modelling, sensitivity and uncertainty analysis. Bioresour Technol 239:353–367. https://doi.org/10.1016/j.biortech.2017.05.018
Mannina G, Cosenza A, Ekama GA (2018) A comprehensive integrated membrane bioreactor model for greenhouse gas emissions. Chem Eng J 334:1563–1572. https://doi.org/10.1016/j.cej.2017.11.061
Massara TM, Solís B, Guisasola A, Katsou E, Baeza JA (2018) Development of an ASM2d-N2O model to describe nitrous oxide emissions in municipal WWTPs under dynamic conditions. Chem Eng J 335:185–196. https://doi.org/10.1016/j.cej.2017.10.119
Jia W, Zhang J, Xie H, Yan Y, Wang J, Zhao Y, Xu X (2012) Effect of PHB and oxygen uptake rate on nitrous oxide emission during simultaneous nitrification denitrification process. Bioresour Technol 113:232–238. https://doi.org/10.1016/j.biortech.2011.10.095
Zhou Y, Lim M, Harjono S, Ng WJ (2012) Nitrous oxide emission by denitrifying phosphorus removal culture using polyhydroxyalkanoates as carbon source. J Environ Sci 24(9):1616–1623. https://doi.org/10.1016/s1001-0742(11)60996-0
Jia W, Liang S, Ngo HH, Guo W, Zhang J, Wang R, Zou Y (2013) Effect of phosphorus load on nutrients removal and N(2)O emission during low-oxygen simultaneous nitrification and denitrification process. Bioresour Technol 141:123–130. https://doi.org/10.1016/j.biortech.2013.02.095
Wang Z, Meng Y, Fan T, Du Y, Tang J, Fan S (2015) Phosphorus removal and N(2)O production in anaerobic/anoxic denitrifying phosphorus removal process: long-term impact of influent phosphorus concentration. Bioresour Technol 179:585–594. https://doi.org/10.1016/j.biortech.2014.12.016
Meyer RL, Zeng RJ, Giugliano V, Blackall LL (2005) Challenges for simultaneous nitrification, denitrification, and phosphorus removal in microbial aggregates: mass transfer limitation and nitrous oxide production. FEMS Microbiol Ecol 52(3):329–338. https://doi.org/10.1016/j.femsec.2004.11.011
Jia W, Liang S, Zhang J, Ngo HH, Guo W, Yan Y, Zou Y (2013) Nitrous oxide emission in low-oxygen simultaneous nitrification and denitrification process: sources and mechanisms. Bioresour Technol 136:444–451. https://doi.org/10.1016/j.biortech.2013.02.117
Henze M, Gujer W, Mino T, van Loosedrecht M (2000) Activated sludge models ASM1, ASM2, ASM2d and ASM3. IWA Publishing, London. https://doi.org/10.2166/9781780402369
Law Y, Ni BJ, Lant P, Yuan Z (2012) N2O production rate of an enriched ammonia-oxidising bacteria culture exponentially correlates to its ammonia oxidation rate. Water Res 46(10):3409–3419. https://doi.org/10.1016/j.watres.2012.03.043
Ni BJ, Yuan Z, Chandran K, Vanrolleghem PA, Murthy S (2012) Evaluating mathematical models for N2O production by ammonia-oxidizing bacteria: towards a unified model. Biotechnol Bioeng 110(1):152–163
Stein LY (2011) Surveying N2O-producing pathways in bacteria. Methods Enzymol 486:131–152. https://doi.org/10.1016/B978-0-12-381294-0.00006-7
Poughon L, Dussap C-g, Gros J-b (2001) Energy model and metabolic flux analysis for autotrophic nitrifiers laurent. Biotechnol Bioeng 72(4):417–433
Zhu X, Chen Y (2011) Reduction of N2O and NO generation in anaerobic-aerobic (low dissolved oxygen) biological wastewater treatment process by using sludge alkaline fermentation liquid. Environ Sci Technol 45(6):2137–2143. https://doi.org/10.1021/es102900h
Berks BC, Ferguson SJ, Moir JWB, Richardson DJ (1995) Enzymes and associated electron transport systems that catalyse the respiratory reduction of nitrogen oxides and oxyanions. BBA 1232(3):97–173. https://doi.org/10.1016/0005-2728(95)00092-5
Yu R, Perez-Garcia O, Lu H, Chandran K (2018) Nitrosomonas europaea adaptation to anoxic-oxic cycling: insights from transcription analysis, proteomics and metabolic network modelling. Sci Total Environ 615:1566–1573. https://doi.org/10.1016/j.scitotenv.2017.09.142
Kim SW, Miyahara M, Fushinobu S, Wakagi T, Shoun H (2010) Nitrous oxide emission from nitrifying activated sludge dependent on denitrification by ammonia-oxidizing bacteria. Bioresour Technol 101(11):3958–3963. https://doi.org/10.1016/j.biortech.2010.01.030
Kester RA, De Boer W, Laanbroek HJ (1997) Production of NO and N2O by pure cultures of nitrifying and denitrifying bacteria during changes in aeration. Appl Environ Microbiol 63(10):3872–3877
Ni BJ, Ruscalleda M, Pellicernacher C, Smets BF (2011) Extension to the general ASM models to include nitrous oxide production via nitrification and denitrification processes. Jaids J Acquir Immune Defic Syndr 6(6):706
Dosta J, Gali A, Benabdallah El-Hadj T, Mace S, Mata-Alvarez J (2007) Operation and model description of a sequencing batch reactor treating reject water for biological nitrogen removal via nitrite. Bioresour Technol 98(11):2065–2075. https://doi.org/10.1016/j.biortech.2006.04.033
Reichert P (1998) AQUASIM 2.0—user manual. Computer program for the identification and simulation of aquatic systems. Swiss Federal Institute for Environmental Science and Technology (EAWAG), Dübendorf. ISBN:3-906484-16-5
Mannina G, Cosenza A, Vanrolleghem PA, Viviani G (2011) A practical protocol for calibration of nutrient removal wastewater treatment models. J Hydroinform 13(4):575–595. https://doi.org/10.2166/hydro.2011.041
Ni BJ, Ye L, Law Y, Byers C, Yuan Z (2013) Mathematical modelling of nitrous oxide (N2O) emissions from full-scale wastewater treatment plants. Environ Sci Technol 47(14):7795–7803. https://doi.org/10.1021/es4005398
Oehmen A, Lemos PC, Carvalho G, Yuan Z, Keller J, Blackall LL, Reis MA (2007) Advances in enhanced biological phosphorus removal: from micro to macro scale. Water Res 41(11):2271–2300. https://doi.org/10.1016/j.watres.2007.02.030
Schuler AJ, Jenkins D (2003) Enhanced biological phosphorus removal from wastewater by biomass with different phosphorus contents, part I: experimental results and comparison with metabolic. Water Environ Res 75(6):512–522. https://doi.org/10.2175/106143003x141303
Chuang SH, Chang WC, Huang YH, Tseng CC, Tai CC (2011) Effects of different carbon supplements on phosphorus removal in low C/P ratio industrial wastewater. Bioresour Technol 102(9):5461–5465. https://doi.org/10.1016/j.biortech.2010.11.118
Mino T, Loosdrecht MCMVAN, Heijnen JJ (1998) Microbiology and biochemistry of the enhanced biological phosphate removal process. Pergramon 32(11):3193–3207
Zhang JPY, Tang X, Wang S (2011) Effect of COD on enhanced biological phosphorus removal system and variation of OUR. Br J Psychiatry 112(483):211–212. https://doi.org/10.1192/bjp.112.483.211-a
Pan Y, Ye L, Ni BJ, Yuan Z (2012) Effect of pH on N(2)O reduction and accumulation during denitrification by methanol utilizing denitrifiers. Water Res 46(15):4832–4840. https://doi.org/10.1016/j.watres.2012.06.003
Wunderlin P, Mohn J, Joss A, Emmenegger L, Siegrist H (2012) Mechanisms of N2O production in biological wastewater treatment under nitrifying and denitrifying conditions. Water Res 46(4):1027–1037. https://doi.org/10.1016/j.watres.2011.11.080
Ding X, Zhao J, Hu B, Chen Y, Ge G, Li X, Wang S, Gao K, Tian X (2016) Mathematical modelling of nitrous oxide production in an anaerobic/oxic/anoxic process. Bioresour Technol 222:39–48. https://doi.org/10.1016/j.biortech.2016.09.092
Acknowledgements
This work was supported by the Natural Science Foundation of China [Nos. 31570568 and 31670585], Science and Technology Planning Project of Guangzhou city, China [Nos. 201607010079, 20160 7020007], Science and Technology Planning Project of Guangdong Province, China [Nos. 2016A020221005, 2017A040405022], National Key Research and Development Project [No. 2018YFE0110400], and the National Natural Science Foundation of China [Nos. 21978102 and 31670585]. The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, Z., Wan, J., Ma, Y. et al. A comprehensive model of N2O emissions in an anaerobic/oxygen-limited aerobic process under dynamic conditions. Bioprocess Biosyst Eng 43, 1093–1104 (2020). https://doi.org/10.1007/s00449-020-02307-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-020-02307-7