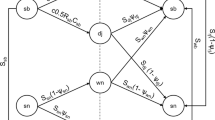
Abstract
Anthropogenic degradation of natural habitats is a global driver of wildlife population declines. Local population responses to such environmental perturbations are generally well understood, but in socially structured populations, interactions between environmental and social factors may influence population responses. Thus, understanding how habitat degradation affects the dynamics of these populations requires simultaneous consideration of social and environmental mechanisms underlying demographic responses. Here we investigated the effect of habitat degradation through commercial forestry on spatiotemporal dynamics of a group-living bird, the Siberian jay, Perisoreus infaustus, in boreal forests of northern Sweden. We assessed the interacting effects of forestry, climate and population density on stage-specific, seasonal life-history rates and population dynamics, using long-term, individual-based demographic data from 70 territories in natural and managed forests. Stage-specific survival and reproductive rates, and consequently population growth, were lower in managed forests than in natural forests. Population growth was most sensitive to breeder survival and was more sensitive to early dispersing juveniles than those delaying dispersal. Forestry decreased population growth in managed forests by reducing reproductive success and breeder survival. Increased snow depth improved winter survival, and warmer spring temperatures enhanced reproductive success, particularly in natural forests. Population growth was stable in natural forests but it was declining in managed forests, and this difference accelerated under forecasted climate scenarios. Thus, climatic change could exacerbate the rate of forestry-induced population decline through reduced snow cover in our study species, and in other species with similar life-history characteristics and habitat requirements.





Similar content being viewed by others
References
Bärring L, Berlin M, Gull BA (2017) Tailored climate indices for climate-proofing operational forestry applications in Sweden and Finland. Int J Climatol 37(1):123–142
Bates, D. et al. (2014) Fitting linear mixed-effects models using lme4
Brownie, C. et al. (1993) Capture-recapture studies for multiple strata including non-Markovian transitions. Biometrics, 1173–1187
Burnham, K.P. (1987) Design and analysis methods for fish survival experiments based on release-recapture, American Fisheries Society
Burnham KP, Anderson DR (2003) Model selection and multimodel inference: a practical information theoretic-approach. Springer Science & Business Media
Calhoun JB (1952) The Social Aspects of Population Dynamics. J Mammal 33:139–159
Caswell H (1989) Analysis of life table response experiments I. Decomposition of effects on population growth rate. Ecol Model 46(3–4):221–237
Caswell, H. (2001) Matrix population models, John Wiley & Sons, Ltd
Caswell H, Trevisan MC (1994) Sensitivity analysis of periodic matrix models. Ecology 75:1299–1303
Cayuela H et al (2014) To breed or not to breed: past reproductive status and environmental cues drive current breeding decisions in a long-lived amphibian. Oecologia 176(1):107–116
Clarke MR, Collins DA, Zucker EL (2002) Responses to Deforestation in a Group of Mantled Howlers in Costa Rica. Int J Primatol 23:365–381
Coker DJ et al (2013) Social group entry rules may limit population resilience to patchy habitat disturbance. Mar Ecol Prog Ser 493:237–242
Crook JH (1970) Social organization and the environment: aspects of contemporary social ethology. Anim Behav 18:197–209
Donati G et al (2011) Better Few than Hungry: flexible Feeding Ecology of Collared Lemurs Eulemur collaris in Littoral Forest Fragments. PLoS ONE 6:e19807
Eggers S, Low M (2014) Differential demographic responses of sympatric Parids to vegetation management in boreal forest. For Ecol Manage 319:169–175
Eggers S et al (2005) Nest predation and habitat change interact to influence Siberian jay numbers. Oikos 111:150–158
Eggers S et al (2006) Predation risk induces changes in nest-site selection and clutch size in the Siberian jay. Proceedings of the Royal Society B: Biological Sciences 273:701–706
Ekman JB, Askenmo CE (1984) Social rank and habitat use in willow tit groups. Anim Behav 32:508–514
Ekman J, Griesser M (2016) Siberian jays: delayed dispersal in the absence of cooperative breeding. Cambridge University Press 6–18:2016
Espmark Y (1964) Studies in dominance-subordination relationship in a group of semi-domestic reindeer (Rangifer tarandus L.). Anim Behav 12:420–426
Esseen PA et al (1997) Boreal Forests. Ecological Bulletins 46:16–47
Gaillard JM, Yoccoz NG (2003) Temporal variation in survival of mammals: a case of environmental canalization? Ecology 84:3294–3306
Garrott RA et al (2003) Climate-induced variation in vital rates of an unharvested large-herbivore population. Can J Zool 81:33–45
Gilroy JJ et al (2008) Could soil degradation contribute to farmland bird declines? Links between soil penetrability and the abundance of yellow wagtails Motacilla flava in arable fields. Biol Cons 141:3116–3126
Griesser M (2013) Do warning calls boost survival of signal recipients? Evidence from a field experiment in a group-living bird species. Frontiers in Zoology 10:1
Griesser M, Lagerberg S (2012) Long-term effects of forest management on territory occupancy and breeding success of an open-nesting boreal bird species, the Siberian jay. For Ecol Manage 271:58–64
Griesser M, Nystrand M (2009) Vigilance and predation of a forest-living bird species depend on large-scale habitat structure. Behavioural Ecology 20:709–715
Griesser M, Nystrand M, Ekman J (2006) Reduced mortality selects for family cohesion in a social species. Proceedings of the Royal Society B: Biological Sciences 273:1881–1886
Griesser M et al (2007) Impact of Forestry Practices on Fitness Correlates and Population Productivity in an Open-Nesting Bird Species. Conserv Biol 21:767–774
Griesser M et al (2012) Causes of ring-related leg injuries in birds–evidence and recommendations from four field studies. PLoS ONE 7:e51891
Griesser M et al (2014) What are the strengths and limitations of direct and indirect assessment of dispersal? Insights from a long-term field study in a group-living bird species. Behav Ecol Sociobiol 68:485–497
Griesser M et al (2015) Fine-scale kin recognition in the absence of social familiarity in the Siberian jay, a monogamous bird species. Mol Ecol 24:5726–5738
Griesser M et al (2017a) Experience buffers extrinsic mortality in a group-living bird species. Oikos 126:1258–1268
Griesser M et al (2017b) Reproductive trade-offs in a long-lived bird species: condition-dependent reproductive allocation maintains female survival and offspring quality. J Evol Biol 30:782–795
Griffiths R et al (1998) A DNA test to sex most birds. Mol Ecol 7:1071–1075
Griffiths JI, Warren PH, Childs DZ (2015) Multiple environmental changes interact to modify species dynamics and invasion rates. Oikos 124:458–468
Hestbeck JB, Nichols JD, Malecki RA (1991) Estimates of movement and site fidelity using mark-resight data of wintering Canada geese. Ecology 72:523–533
Imbeau L, Mönkkönen M, Desrochers A (2001) Long-Term Effects of Forestry on Birds of the Eastern Canadian Boreal Forests: a comparison with Fennoscandia. Conserv Biol 15:1151–1162
Jetz W et al (2014) Global distribution and conservation of evolutionary distinctness in birds. Curr Biol 24:919–930
Jiguet F et al (2007) Climate envelope, life history traits and the resilience of birds facing global change. Glob Change Biol 13:1672–1684
Laake JL (2013) RMark: An R Interface for Analysis of Capture-Recapture Data with MARK. AFSC Processed Rep 2013–01. Alaska Fish. Sci. Cent., NOAA, Natl. Mar. Fish. Serv., 7600 Sand Point Way NE, Seattle WA 98115
Li Z, Wang Z, Ge C (2013) Time Budgets of Wintering Red-Crowned Cranes: effects of Habitat, Age and Family Size. Wetlands 33:227–232
Linder P, Östlund L (1998) Structural changes in three mid-boreal Swedish forest landscapes, 1885–1996. Biol Cons 85:9–19
Lui, C. (2010) Survival in a population of Siberian jays. Master’s thesis. Uppsala University
Mac Nally R et al (2009) Collapse of an avifauna: climate change appears to exacerbate habitat loss and degradation. Divers Distrib 15:720–730
Mantyka-pringle CS, Martin TG, Rhodes JR (2012) Interactions between climate and habitat loss effects on biodiversity: a systematic review and meta-analysis. Glob Change Biol 18:1239–1252
Mielikäinen K, Hynynen J (2003) Silvicultural management in maintaining biodiversity and resistance of forests in Europe–boreal zone: case Finland. J Environ Manage 67:47–54
Nystrand M et al (2010) Habitat-specific demography and source–sink dynamics in a population of Siberian jays. J Anim Ecol 79:266–274
Opdam P, Wascher D (2004) Climate change meets habitat fragmentation: linking landscape and biogeographical scale levels in research and conservation. Biol Cons 117:285–297
Ozgul A et al (2014) Linking body mass and group dynamics in an obligate cooperative breeder. J Anim Ecol 83:1357–1366
Peterson SL et al (2014) Legacy effects of habitat degradation by Lesser Snow Geese on nesting Savannah Sparrows. The Condor 116:527–537
Powell LA (2007) Approximating variance of demographic parameters using the delta method: a reference for avian biologists. The Condor 109:949–954
R Core Team (2016) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Räisänen J (2016) Twenty-first century changes in snowfall climate in Northern Europe in ENSEMBLES regional climate models. Clim Dyn 46(1–2):339–353
Rotella JJ et al (2012) Evaluating the demographic buffering hypothesis with vital rates estimated for Weddell seals from 30 years of mark–recapture data. J Anim Ecol 81:162–173
Stearns SC (1992) The evolution of life histories. Oxford University Press, Oxford
Steyaert SM et al (2013) Male reproductive strategy explains spatiotemporal segregation in brown bears. J Anim Ecol 82:836–845
Thingstad PG, Skjeggedal T, Markhus G (2003) Human-induced alteration of two boreal forest landscapes in central Norway, and some possible consequences for avian fauna. Journal for Natural Conservation 11:157–170
Townsend HM, Anderson DJ (2007) Assessment of costs of reproduction in a pelagic seabird using multistate mark–recapture models. Evolution 61(8):1956–1968
Warkentin IG, Reed JM, Dunham SM (2004) Offspring size as an index of habitat degradation. Ornithological Science 3:145–153
Warren MS et al (2001) Rapid responses of British butterflies to opposing forces of climate and habitat change. Nature 414:65–69
Widén, P. (1987) Goshawk predation during winter, spring and summer in a boreal forest area of central Sweden. Holarctic Ecology, 104–109
Wiktander U, Olsson O, Nilsson SG (2000) Parental care and social mating system in the Lesser Spotted Woodpecker Dendrocopos minor. J Avian Biol 31:447–456
Wiley, R.H. (1974) Evolution of social organization and life-history patterns among grouse. The Quarterly Review of Biology, 201–227
Williams, B.K., Nichols, J.D. & Conroy, M.J. (2002) Analysis and management of animal populations. Academic Press
Wisdom MJ, Mills LS, Doak DF (2000) Life stage simulation analysis: estimating vital-rate effects on population growth for conservation. Ecology 81(3):628–641
White GC, Burnham KP (1999) Program MARK: survival estimation from populations of marked animals. Bird study 46(sup1):S120–S139
Acknowledgements
We thank Chloe Nater for help with analysis; Folke Lindgren, Jan Ekman, Bohdan Sklepkovych, Sönke Eggers, Magdalena Nystrand, Jonathan Barnaby, Xenia Schleuning, Julian Klein and all field volunteers for collecting the field data; and Susanne Schindler, Christophe Bousquet and Agnes Olin for comments on the manuscript. NetOne provided us the much-needed internet access during the fieldwork. This study has been supported by grants from the Swiss National Science Foundation (MG: PPOOP3_123520, PP00P3_150752), ERA-Net BiodivERsA (AO, MG: 31BD30_172465), the Swedish Research Council (MG, Jan Ekman), Formas (Jan Ekman), the National Science Centre, Poland, through the European Union’s Horizon 2020 research and innovation program (Marie Sklodowska-Curie Grant 665778 (MG), and University of Zurich (AO, MG).
Author information
Authors and Affiliations
Contributions
MG collected the data; KLM and AO analysed the data; KLM wrote the manuscript; AO and MG assisted in writing and revising the manuscript.
Corresponding author
Ethics declarations
Data accessibility
Data used in this study will be submitted to Dryad.
Additional information
Communicated by David N Koons.
We show how two anthropogenic drivers of environmental concern affect population dynamics in temperate forests and the implications of heterogeneous responses of individuals of differing social rank.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Layton-Matthews, K., Ozgul, A. & Griesser, M. The interacting effects of forestry and climate change on the demography of a group-living bird population. Oecologia 186, 907–918 (2018). https://doi.org/10.1007/s00442-018-4100-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-018-4100-z