Abstract
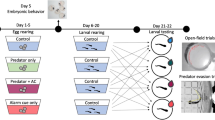
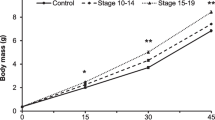
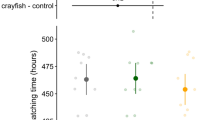
Carry-over effects influence trait responses in later life stages as a result of early experience with environmental cues. Predation risk is an influential stressor and selection exists for early recognition of threats. In particular, invasive species may benefit from carry-over effects by preemptively recognizing and responding to novel predators via latent developmental changes and embryonic learning. In a factorial experiment, we conditioned invasive American bullfrog embryos (Lithobates catesbeianus) to the odor of a novel fish predator, largemouth bass (Micropterus salmoides) alone or in combination with injured conspecific cues. We quantified developmental carryover in the larval life stage and found that individuals conditioned to the highest risk (fish and injured conspecific cues) grew into longer bodied larvae relative to larvae from lower risk treatments. We also assessed embryonic learning, a behavioral carry-over effect, and found an interaction between embryonic conditioning and larval exposure. Behavioral responses were only found in scenarios when predation risk varied in intensity across life history stages, thus requiring a more flexible antipredator strategy. This indicates a potential trade-off between the two strategies in larval growth and development rates, and time until metamorphosis. Our results suggest that early predator exposure and carry-over effects have significant impacts on life history trajectories for American bullfrogs. This research contributes to our understanding of a potentially important invasion mechanism in an anuran species of conservation concern.




Similar content being viewed by others
References
Atherton JA, McCormick MI (2015) Active in the sac: damselfish embryos use innate recognition of odours to learn predation risk before hatching. Anim Behav 103:1–6. doi:10.1016/j.anbehav.2015.01.033
Benard MF, Fordyce JA (2003) Are induced defenses costly? Consequences of predator-induced defenses in western toads, Bufo boreas. Ecology 84:68–78. doi:10.1890/0012-9658(2003)084[0068:AIDCCO]2.0.CO;2
Blumstein DT (2016) Habituation and sensitization: new thoughts about old ideas. Anim Behav 120:255–262. doi:10.1016/j.anbehav.2016.05.012
Brown GE, Smith RJF (1998) Acquired predator recognition in juvenile rainbow trout (Oncorhynchus mykiss): conditioning hatchery-reared fish to recognize chemical cues of a predator. Can J Fish Aquat Sci 55:611–617. doi:10.1139/cjfas-55-3-611
Chivers DP, Wisenden BD, Smith R, Jan F (1996) Damselfly larvae learn to recognize predators from chemical cues in the predator’s diet. Anim Behav 52:315–320. doi:10.1006/anbe.1996.0177
DeWitt TJ (1998) Costs and limits of phenotypic plasticity: tests with predator-induced morphology and life history in a freshwater snail. J Evol Biol 11:465. doi:10.1007/s000360050100
Epp KJ, Gabor CR (2008) Innate and learned predator recognition mediated by chemical signals in Eurycea nana. Ethology 114:607–615. doi:10.1111/j.1439-0310.2008.01494.x
Ferland-Raymond B, March RE, Metcalfe CD, Murray DL (2010) Prey detection of aquatic predators: assessing the identity of chemical cues eliciting prey behavioral plasticity. Biochem Syst Ecol 38:169–177. doi:10.1016/j.bse.2009.12.035
Ferrari MCO, Chivers DP (2009a) Temporal variability, threat sensitivity and conflicting information about the nature of risk: understanding the dynamics of tadpole antipredator behaviour. Anim Behav 78:11–16. doi:10.1016/j.anbehav.2009.03.016
Ferrari MCO, Chivers DP (2009b) Sophisticated early life lessons: threat-sensitive generalization of predator recognition by embryonic amphibians. Behav Ecol 20:1295–1298. doi:10.1093/beheco/arp135
Ferrari MCO, Chivers DP (2009c) Latent inhibition of predator recognition by embryonic amphibians. Biol Lett 5:160–162. doi:10.1098/rsbl.2008.0641
Ferrari MCO, Chivers DP (2010) The ghost of predation future: threat-sensitive and temporal assessment of risk by embryonic woodfrogs. Behav Ecol Sociobiol 64:549–555. doi:10.1007/s00265-009-0870-y
Ferrari MCO, Chivers DP (2013) Adaptive responses of embryonic amphibians to predation risk. In: East ML, Dehnhard M (eds) Chemical signals in vertebrates, 12th edn. Springer, New York, pp 229–244
Ferrari MCO, Manek AK, Chivers DP (2010a) Temporal learning of predation risk by embryonic amphibians. Biol Lett 6:308–310. doi:10.1098/rsbl.2009.0798
Ferrari MCO, Wisenden BD, Chivers DP (2010b) Chemical ecology of predator–prey interactions in aquatic ecosystems: a review and prospectus. The present review is one in the special series of reviews on animal–plant interactions. Can J Zool 88:698–724. doi:10.1139/Z10-029
Ferrari MCO, Brown GE, Messier F, Chivers DP (2009) Threat-sensitive generalization of predator recognition by larval amphibians. Behav Ecol Sociobiol 63:1369–1375
Ferrari MCO, McCormick MI, Allan BJM et al (2015) Living in a risky world: the onset ond ontogeny of an integrated antipredator phenotype in a coral reef fish. Sci Rep 5:15537
Fox J, Weisberg S (2011) An R companion to applied regression, 2nd edn. Sage, Thousand Oaks
Garcia TS, Stacy J, Sih A (2004) Larval salamander response to UV radiation and predation risk: color change and microhabitat use. Ecol Appl 14:1055–1064
Garcia TS, Thurman LL, Rowe JC, Selego SM (2012) Antipredator behavior of American Bullfrogs (Lithobates catesbeianus) in a novel environment. Ethology 118:867–875. doi:10.1111/j.1439-0310.2012.02074.x
Gosner KL (1960) A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica 16:183–190
Hay ME (2009) Marine chemical ecology: chemical signals and cues structure marine populations, communities, and ecosystems. Ann Rev Mar Sci 1:193–212. doi:10.1146/annurev.marine.010908.163708
Hayes MP, Jennings MR (1986) Decline of ranid frog species in Western North America: are Bullfrogs (Rana catesbeiana) responsible? J Herpetol 20:490–509
Hemmi JM, Merkle T (2009) High stimulus specificity characterizes anti-predator habituation under natural conditions. Proc R Soc B Biol Sci 276:4381–4388. doi:10.1098/rspb.2009.1452
Hettyey A, Tóth Z, Thonhauser KE et al (2015) The relative importance of prey-borne and predator-borne chemical cues for inducible antipredator responses in tadpoles. Oecologia 179:699–710. doi:10.1007/s00442-015-3382-7
Hettyey A, Thonhauser KE, Bókony V et al (2016) Naive tadpoles do not recognize recent invasive predatory fishes as dangerous. Ecology 97:2975–2985. doi:10.1002/ecy.1532
Jacobsen HP, Stabell OB (2004) Antipredator behaviour mediated by chemical cues: the role of conspecific alarm signalling and predator labelling in the avoidance response of a marine gastropod. Oikos 104:43–50. doi:10.1111/j.0030-1299.2004.12369.x
Lever C (2003) Naturalized reptiles and amphibians of the world. Oxford University Press, Oxford and New York
Lima SL, Dill LM (1990) Behavioral decisions made under the risk of predation: a review and prospectus. Can J Zool 68:619–640. doi:10.1139/z90-092
Lima SL, Bednekoff PA (1999) Temporal Variation in Danger Drives Antipredator Behavior: the Predation Risk Allocation Hypothesis. Am Nat 153:649–659
Lowe C, Browne S, Boudjelas M, De SM (2000) 100 of the world’s worst invasive alien species: a selection from the global invasive species database. Aliens 12:12
Marshall D, Pechenik J, Keough M (2003) Larval activity levels and delayed metamorphosis affect post-larval performance in the colonial ascidian Diplosoma listerianum. Mar Ecol Prog Ser 246:153–162. doi:10.3354/meps246153
Mathis A, Ferrari MC, Windel N et al (2008) Learning by embryos and the ghost of predation future. Proc R Soc B Biol Sci 275:2603–2607. doi:10.1098/rspb.2008.0754
McCarthy TM, Fisher WA (2000) Multiple predator-avoidance behaviours of the freshwater snail Physella heterostropha pomila: responses vary with risk. Freshw Biol 44:387–397. doi:10.1046/j.1365-2427.2000.00576.x
McIntyre PB, Baldwin S, Flecker AS (2004) Effects of behavioral and morphological plasticity on risk of predation in a Neotropical tadpole. Oecologia 141:130–138. doi:10.1007/s00442-004-1652-x
Mitchell MD, McCormick MI (2013) Ontogenetic differences in chemical alarm cue production determine antipredator responses and learned predator recognition. Behav Ecol Sociobiol 67:1123–1129. doi:10.1007/s00265-013-1537-2
Nelson AB, Alemadi SD, Wisenden BD (2013) Learned recognition of novel predator odour by convict cichlid embryos. Behav Ecol Sociobiol 67:1269–1273. doi:10.1007/s00265-013-1554-1
Pearl CA, Adams MJ, Bury RB, McCreary B (2004) Asymmetrical Effects of Introduced Bullfrogs (Rana catesbeiana) on native Ranid Frogs in Oregon. Copeia 2004:11–20
Pechenik JA (2006) Larval experience and latent effects—metamorphosis is not a new beginning. Integr Comp Biol 46:323–333. doi:10.1093/icb/icj028
Pintor LM, Byers JE (2015) Do native predators benefit from non-native prey? Ecol Lett 18:1174–1180. doi:10.1111/ele.12496
Polo-Cavia N, Gomez-Mestre I (2014) Learned recognition of introduced predators determines survival of tadpole prey. Funct Ecol 28:432–439. doi:10.1111/1365-2435.12175
Pujol-Buxó E, San Sebastián O, Garriga N, Llorente GA (2013) How does the invasive/native nature of species influence tadpoles’ plastic responses to predators? Oikos 122:19–29. doi:10.1111/j.1600-0706.2012.20617.x
R Core Team (2015) R: A Language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Relyea RA (2001) Morphological and behavioral plasticity of larval anurans in response to different predators. Ecology 82:523–540
Relyea RA (2004) Fine-tuned phenotypes: tadpole plasticity under 16 combinations of predators and competitors. Ecol Ecol 85:172–179
Relyea RA, Werner EE (1999) Quantifying the relation between predator-induced behavior and growth performance in larval anurans. Ecology 80:2117–2124
Richardson JML (2001) A comparative study of activity levels in larval anurans and response to the presence of different predators. Behav Ecol 12:51–58. doi:10.1093/oxfordjournals.beheco.a000378
Rodriguez-Cabal MA, Williamson M, Simberloff D (2013) Overestimation of establishment success of non-native birds in Hawaii and Britain. Biol Invasions 15:249–252. doi:10.1007/s10530-012-0285-y
Roff DA (1992) The evolution of life histories: theory and analysis. Chapman and Hall, New York
Sih A, Moore R (1993) Delayed hatching of salamander eggs in response to enhanced larval predation risk on JSTOR. Am Nat 142:947–960
Sih A, Bolnick DI, Luttbeg B et al (2010) Predator-prey naïveté, antipredator behavior, and the ecology of predator invasions. Oikos 119:610–621. doi:10.1111/j.1600-0706.2009.18039.x
Skelly DK, Werner EE (1990) Behavioral and life-historical responses of larval American Toads to an odonate predator. Ecology 71:2313–2322. doi:10.2307/1938642
Smith DC, Van Buskirk J (1995) Phenotypic design, plasticity, and ecological performance in two tadpole species. Source Am Nat Am Nat 145:211–233
Tarvin RD, Silva Bermúdez C, Briggs VS, Warkentin KM (2015) Carry-over effects of size at metamorphosis in red-eyed treefrogs: higher survival but slower growth of larger metamorphs. Biotropica 47:218–226. doi:10.1111/btp.12198
Tollrian R, Harvell CD (1999) The ecology and evolution of inducible defenses. Princeton University Press, Princeton
van Allen BG, Briggs VS, McCoy MW, Vonesh JR (2010) Carry-over effects of the larval environment on post-metamorphic performance in two hylid frogs. Oecologia 164:891–898. doi:10.1007/s00442-010-1728-8
Van Buskirk J (2000) The costs of an inducible defence in anuran larvae. Ecol 81:2813–2821
Van Buskirk J (2002) A comparative test of the adaptive plasticity hypothesis: relationships between habitat and phenotype in Anuran Larvae. Am Nat 160:87–102. doi:10.1086/340599
Van Buskirk J, McCollum SA (2000) Influence of tail shape on tadpole swimming performance. J Exp Biol 203:2149–2158
Van Buskirk J, Schmidt BR (2000) Predator-induced phenotypic plasticity in larval newts: trade-offs, selection, and variation in nature. Ecol 81:3009–3028
Warkentin KM (1995) Adaptive plasticity in hatching age: a response to predation risk trade-offs. Proc Natl Acad Sci 92:3507–3510. doi:10.1073/pnas.92.8.3507
Warkentin KM (2007) Oxygen, gills, and embryo behavior: mechanisms of adaptive plasticity in hatching. Comp Biochem Physiol Part A Mol Integr Physiol 148:720–731. doi:10.1016/j.cbpa.2007.02.009
Werner EE (1986) Amphibian metamorphosis: growth rite, predation risk and the optimal size at transformation. Am Nat 128:319–341
Wickham H (2009) ggplot2: elegant graphics for data analysis. Springer, New York. doi:10.1007/978-0-387-98141-3
Wilbur HM, Collins JP (1973) Ecological aspects of Amphibian Metamorphosis: nonnormal distributions of competitive ability reflect selection for facultative metamorphosis. Science 182:1305–1314. doi:10.1126/science.182.4119.1305
Williamson MH, Brown KC, Holdgate MW et al (1986) The analysis and modelling of british invasions. Philos Trans R Soc B Biol Sci 314:505–522. doi:10.1098/rstb.1986.0070
Wilson RS, Kraft PG, Van Damme R (2005) Predator-specific changes in the morphology and swimming performance of larval Rana lessonae. Funct Ecol 19:238–244. doi:10.1111/j.1365-2435.2005.00958.x
Winandy L, Denoël M (2013) Cues from Introduced fish alter shelter use and feeding behaviour in adult Alpine Newts. Ethology 119:121–129. doi:10.1111/eth.12043
Acknowledgements
We would like to acknowledge Angie Soken, Randy Wildman, Dave Paoletti and Greyson Paoletti for help with animal collection. Danielle Nelson and Lindsey Thurman assisted with experimental set-up and animal care. Animals were collected under Oregon Department of Fisheries and Wildlife Service Special Use Permit No. 008-14 and Oregon State University Animal Care and Use Protocol No. 4575.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Joel trexler.
Rights and permissions
About this article
Cite this article
Garcia, T.S., Urbina, J.C., Bredeweg, E.M. et al. Embryonic learning and developmental carry-over effects in an invasive anuran. Oecologia 184, 623–631 (2017). https://doi.org/10.1007/s00442-017-3905-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-017-3905-5