Abstract
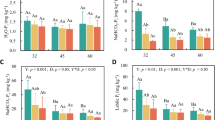
How forests cope with drought-induced perturbations and how the dependence of soil respiration on environmental and biological drivers is affected in a warming and drying context are becoming key questions. The aims of this study were to determine whether drought-induced die-off and forest succession were reflected in soil respiration and its components and to determine the influence of climate on the soil respiration components. We used the mesh exclusion method to study seasonal variations in soil respiration (R S) and its components: heterotrophic (R H) and autotrophic (R A) [further split into fine root (R R) and mycorrhizal respiration (R M)] in a mixed Mediterranean forest where Scots pine (Pinus sylvestris L.) is undergoing a drought-induced die-off and is being replaced by holm oak (Quercus ilex L.). Drought-induced pine die-off was not reflected in R S nor in its components, which denotes a high functional resilience of the plant and soil system to pine die-off. However, the succession from Scots pine to holm oak resulted in a reduction of R H and thus in an important decrease of total respiration (R S was 36 % lower in holm oaks than in non-defoliated pines). Furthermore, R S and all its components were strongly regulated by soil water content-and-temperature interaction. Since Scots pine die-off and Quercus species colonization seems to be widely occurring at the driest limit of the Scots pine distribution, the functional resilience of the soil system over die-off and the decrease of R S from Scots pine to holm oak could have direct consequences for the C balance of these ecosystems.







Similar content being viewed by others
References
Aguadé D, Poyatos R, Rosas T, Martínez-Vilalta J (2015) Comparative drought responses of Quercus ilex L. and Pinus sylvestris L. in a montane forest undergoing a vegetation shift. Forests 6:2505–2529
Allen CD, Macalady AK, Chenchouni H et al (2010) A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For Ecol Manage 259:660–684
Allen CD, Breshears DD, McDowell NG (2015) On underestimation of global vulnerability to tree mortality and forest die-off from hotter drought in the Anthropocene. Ecosphere 6(8):1–55 (art129)
Amiro BD, Barr AG, Barr JG et al (2010) Ecosystem carbon dioxide fluxes after disturbance in forests of North America. J Geophys Res 115:G00K02. doi:10.1029/2010JG001390
Anderegg WRL, Kane JM, Anderegg LDL (2013) Consequences of widespread tree mortality triggered by drought and temperature stress. Nat Clim Chang 3:30–36
Asensio D, Peñuelas J, Ogaya R, Llusia J (2007) Seasonal soil and leaf CO2 exchange rates in a Mediterranean holm oak forest and their responses to drought conditions. Atmos Environ 41:2447–2455
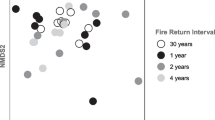
Barba J, Curiel Yuste J, Martínez-Vilalta J, Lloret F (2013) Drought-induced tree species replacement is reflected in the spatial variability of soil respiration in a mixed Mediterranean forest. For Ecol Manage 306:79–87
Barba J, Lloret F, Curiel Yuste J (2015) Effects of drought-induced forest die-off on litter decomposition. Plant Soil. doi:10.1007/s11104-015-2762-4
Bartón K (2014) MuMIn: multi-model inference. R package version 3.1-96
Binkley D, Giardina C (1998) Why do tree species affect soils? The warp and woof of tree-soil interactions. Biogeochemistry 42:89–106
Binkley D, Stape JL, Takahashi EN, Ryan MG (2006) Tree-girdling to separate root and heterotrophic respiration in two Eucalyptus stands in Brazil. Oecologia 148:447–454
Borkhuu B, Peckham SD, Ewers BE et al (2015) Does soil respiration decline following bark beetle induced forest mortality? Evidence from a lodgepole pine forest. Agric For Meteorol 214–215:201–207
Brown M, Black TA, Nesic Z et al (2010) Impact of mountain pine beetle on the net ecosystem production of lodgepole pine stands in British Columbia. Agric For Meteorol 150:254–264
Carnicer J, Coll M, Ninyerola M et al (2011) Widespread crown condition decline, food web disruption, and amplified tree mortality with increased climate change-type drought. Proc Natl Acad Sci USA 108:1474–1478
Carnicer J, Coll M, Pons X et al (2014) Large-scale recruitment limitation in Mediterranean pines: the role of Quercus ilex and forest successional advance as key regional drivers. Glob Ecol Biogeogr 23:371–384
Cornwell WK, Cornelissen JHC, Amatangelo K et al (2008) Plant species traits are the predominant control on litter decomposition rates within biomes worldwide. Ecol Lett 11:1065–1071
Curiel Yuste J, Barba J, Fernandez-Gonzalez AJ et al (2012) Changes in soil bacterial community triggered by drought-induced gap succession preceded changes in soil C stocks and quality. Ecol Evol 2:3016–3031
Curiel Yuste J, Fernandez-Gonzalez AJ, Fernandez-Lopez M et al (2014) Strong functional stability of soil microbial communities under semiarid Mediterranean conditions and subjected to long-term shifts in baseline precipitation. Soil Biol Biochem 69:223–233
Dixon RK, Solomon AM, Brown SE et al (1994) Carbon pools and flux of global forest ecosystems. Science (80) 263:185–90
Edburg SL, Hicke JA, Brooks PD et al (2012) Cascading impacts of bark beetle-caused tree mortality on coupled biogeophysical and biogeochemical processes. Front Ecol Environ 10:416–424
Ek H (1997) The influence of nitrogen fertilization on the carbon economy of Paxillus involutus in ectomycorrhizal association with Betula pendula. New Phytol 135:133–142
Galiano L, Martínez-Vilalta J, Lloret F (2010) Drought-induced multifactor decline of Scots pine in the Pyrenees and potential vegetation change by the expansion of co-occurring oak species. Ecosystems 13:978–991
Galiano L, Martínez-Vilalta J, Eugenio M et al (2013) Seedling emergence and growth of Quercus spp. following severe drought effects on a Pinus sylvestris canopy. J Veg Sci 24:580–588
Gough CM, Hardiman BS, Nave LE et al (2013) Sustained carbon uptake and storage following moderate disturbance in a Great Lakes forest. Ecol Appl 23:1202–1215
Heinemeyer A, Hartley IP, Evans SP et al (2007) Forest soil CO2 flux: uncovering the contribution and environmental responses of ectomycorrhizas. Glob Chang Biol 13:1786–1797
Hereş AM, Martínez-Vilalta J, Claramunt López B (2012) Growth patterns in relation to drought-induced mortality at two Scots pine (Pinus sylvestris L.) sites in NE Iberian Peninsula. Trees 26:621–630
Hereter A, Sánchez JR (1999) Experimental areas of Prades and Montseny. In: Rodà F, Retana J, Gracia CA, Bellot J (eds) Ecology of Mediterranean evergreen oak forests. Springer, Berlin, pp 15–27
Högberg P, Read DJ (2006) Towards a more plant physiological perspective on soil ecology. Trends Ecol Evol 21:548–554
Högberg P, Nordgren A, Buchmann N et al (2001) Large-scale forest girdling shows that current photosynthesis drives soil respiration. Nature 411:789–792
Högberg P, Löfvenius MO, Nordgren A (2009) Partitioning of soil respiration into its autotrophic and heterotrophic components by means of tree-girdling in old boreal spruce forest. For Ecol Managee 257:1764–1767
Jackson RB, Mooney HA, Schulze ED (1997) A global budget for fine root biomass, surface area, and nutrient contents. Proc Natl Acad Sci USA 94:7362–7366
Janssens IA, Lankreijer H, Matteucci G et al (2001) Productivity overshadows temperature in determining soil and ecosystem respiration across European forests. Glob Chang Biol 7:269–278
Keenan T, García R, Friend AD et al (2009) Improved understanding of drought controls on seasonal variation in Mediterranean forest canopy CO2 and water fluxes through combined in situ measurements and ecosystem modelling. Biogeosciences 6:1423–1444
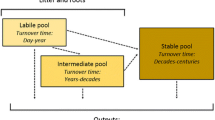
Kuzyakov Y (2006) Sources of CO2 efflux from soil and review of partitioning methods. Soil Biol Biochem 38:425–448
Levy-Varon JH, Schuster WSF, Griffin KL (2012) The autotrophic contribution to soil respiration in a northern temperate deciduous forest and its response to stand disturbance. Oecologia 169:211–220
Levy-Varon JH, Schuster WSF, Griffin KL (2014) Rapid rebound of soil respiration following partial stand disturbance by tree girdling in a temperate deciduous forest. Oecologia 174:1415–1424
Lloret F, Escudero A, Iriondo JM et al (2012) Extreme climatic events and vegetation: the role of stabilizing processes. Glob Chang Biol 18:797–805
Malhi Y, Baldocchi DD, Jarvis PG (1999) The carbon balance of tropical, temperate and boreal forests. Plant Cell Environ 22:715–740
Martínez-Vilalta J, Piñol J (2002) Drought-induced mortality and hydraulic architecture in pine populations of the NE Iberian Peninsula. For Ecol Manage 161:247–256
Martínez-Vilalta J, Aguadé D, Banqué M et al (2012) Las poblaciones ibéricas de pino albar ante el cambio climático: con la muerte en los talones. Rev Ecosist 21:15–21
Moore DJP, Trahan NA, Wilkes P et al (2013) Persistent reduced ecosystem respiration after insect disturbance in high elevation forests. Ecol Lett 16:731–737
Moyano FE, Atkin OK, Bahn M et al (2010) Respiration from roots to mycorrhizosphere. In: Kutsch WL, Bahn M, Heinemeyer A (eds) Soil carbon dynamics. An integrated methodology, 1st edn. Cambridge University Press, Cambridge, pp 127–156
Nave LE, Gough CM, Maurer KD et al (2011) Disturbance and the resilience of coupled carbon and nitrogen cycling in a north temperate forest. J Geophys Res 116:G04016. doi:10.1029/2011JG001758
Ninyerola M, Pons X, Roure JM (2007a) Objective air temperature mapping for the Iberian Peninsula using spatial interpolation and GIS. Int J Climatol 27:1231–1242
Ninyerola M, Pons X, Roure JM (2007b) Monthly precipitation mapping of the Iberian Peninsula using spatial interpolation tools implemented in a geographic information system. Theor Appl Climatol 89:195–209
Palacio S, Maestro M, Montserrat-Martí G (2007) Seasonal dynamics of non-structural carbohydrates in two species of Mediterranean sub-shrubs with different leaf phenology. Environ Exp Bot 59:34–42
Peñuelas J, Lloret F, Montoya R (2001) Severe drought effects on Mediterranean woody flora in Spain. For Sci 47:214–218
Pereira-Blanco E (2014) Response of fine root respiration to variations in biotic and abiotic factors in a mixed Mediterranean forest affected by drought induced secondary succession. Universitat Autònoma de Barcelona, Barcelona
Piao S, Luyssaert S, Ciais P et al (2010) Forest annual carbon cost: a global-scale analysis of autotrophic respiration. Ecology 91:652–661
Pinheiro J, Bates D, DepRoy S (2009) Linear and nonlinear mixed effects models. R package version 3.1-96
Poyatos R, Aguadé D, Galiano L et al (2013) Drought-induced defoliation and long periods of near-zero gas exchange play a key role in accentuating metabolic decline of Scots pine. New Phytol 200:388–401
Redding T, Winkler R, Teti P et al (2008) Mountain pine beetle and watershed hydrology. BC J Ecosyst Manage 9:33–50
Reed DE, Ewers BE, Pendall E (2014) Impact of mountain pine beetle induced mortality on forest carbon and water fluxes. Environ Res Lett 9:105004
Reichstein M, Tenhunen JD, Roupsard O et al (2002) Severe drought effects on ecosystem CO2 and H2O fluxes at three Mediterranean evergreen sites: revision of current hypotheses? Glob Chang Biol 8:999–1017
Reichstein M, Bahn M, Ciais P et al (2013) Climate extremes and the carbon cycle. Nature 500:287–295
Rey A, Pegoraro E, Tedeschi V et al (2002) Annual variation in soil respiration and its components in a coppice oak forest in Central Italy. Glob Chang Biol 8:851–866
Ruehr NK, Buchmann N (2010) Soil respiration fluxes in a temperate mixed forest: seasonality and temperature sensitivities differ among microbial and root-rhizosphere respiration. Tree Physiol 30:165–176
Stocker TF, Qin D, Plattner GK et al (eds) (2013) IPCC, 2013: climate change 2013: the physical science basis. Contribution of Working Group I to the fifth assessment report of the Intergovernmental Panel on Climate Change
Strickland MS, Lauber C, Fierer N, Bradford MA (2009) Testing the functional significance of microbial community composition. Ecology 90:441–451
Subke J-A, Inglima I, Francesca Cotrufo M (2006) Trends and methodological impacts in soil CO2 efflux partitioning: a metaanalytical review. Glob Chang Biol 12:921–943
Subke J-A, Voke NR, Leronni V et al (2011) Dynamics and pathways of autotrophic and heterotrophic soil CO2 efflux revealed by forest girdling. J Ecol 99:186–193
Sus O, Poyatos R, Barba J et al (2014) Time variable hydraulic parameters improve the performance of a mechanistic stand transpiration model. A case study of Mediterranean Scots pine sap flow data assimilation. Agric For Meteorol 198–199:168–180
Tang J, Baldocchi DD, Xu L (2005) Tree photosynthesis modulates soil respiration on a diurnal time scale. Glob Chang Biol 11:1298–1304
Tedeschi V, Rey A, Manca G et al (2006) Soil respiration in a Mediterranean oak forest at different developmental stages after coppicing. Glob Chang Biol 12:110–121
Uren NC (2000) Types, amounts, and possible functions of compounds released into the rhizosphere by soil-grown plants. In: Pinton R, Varanini Z, Nannipieri P (eds) The rhizosphere: biochemistry and organic substances at the soil–plant interface, 2nd edn. Dekker, New York, pp 19–40
Vilà-Cabrera A, Martínez-Vilalta J, Galiano L, Retana J (2013) Patterns of forest decline and regeneration across Scots pine populations. Ecosystems 16:323–335
Vivanco L, Austin AT (2008) Tree species identity alters forest litter decomposition through long-term plant and soil interactions in Patagonia, Argentina. J Ecol 96:727–736
Acknowledgments
The authors thank I. Azcoitia, G. Barba, J. Estrada, I. Ourêlo, P. Pellicer and I. Urbina for their help in fieldwork and S. Vicca for her valuable comments. The insights from two reviewers helped to improve the manuscript. This study was supported by the Spanish government projects SECASOL (CGL2009-08101), DRIM (CGL2010-16373), VULGLO (CGL2010-22180-C03-03), SECADIN (CGL2012-32965) and VERONICA (CGL2013-42271-P); by the Government of Catalonia grants (2009-SGR-00247 and 2014-SGR-453); and by a Community of Madrid grant REMEDINAL 2 (CM S2009/AMB-1783). J. B. was supported by FPI (BES-2010-036558) and EEBB (EEBB-I-13-07002) scholarships from the Spanish Ministry of Economy and Competitiveness.
Author contribution statement
J. B., J. C. Y. and F. L. L. conceived and designed the experiment; J. B., J. C. Y. and R. P. performed the experiment; J. B., J. C. Y., I. J. and R. P. analysed the data; J. B. wrote the paper and all authors edited the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by David Tissue.
Globally increasing drought-induced forest die-off and its associated vegetation shifts may have direct impacts on soil respiration. Here we found that soil respiration and its autotrophic and heterotrophic components remained unaffected 3–11 years following drought-induced Scots pine die-off. Despite this post-disturbance functional resilience the replacement of Scots pine by holm oak was associated with a strong reduction in the heterotrophic respiration component producing an important drop in total soil respiration.
Rights and permissions
About this article
Cite this article
Barba, J., Curiel Yuste, J., Poyatos, R. et al. Strong resilience of soil respiration components to drought-induced die-off resulting in forest secondary succession. Oecologia 182, 27–41 (2016). https://doi.org/10.1007/s00442-016-3567-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-016-3567-8