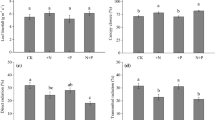
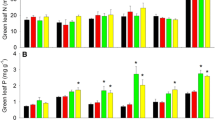
Abstract
Boreal coniferous forests are characterized by fairly open canopies where understory vegetation is an important component of ecosystem C and N cycling. We used an ecophysiological approach to study the effects of N additions on uptake and partitioning of C and N in two dominant understory shrubs: deciduous Vaccinium myrtillus in a Picea abies stand and evergreen Vaccinium vitis-idaea in a Pinus sylvestris stand in northern Sweden. N was added to these stands for 16 and 8 years, respectively, at rates of 0, 12.5, and 50 kg N ha−1 year−1. N addition at the highest rate increased foliar N and chlorophyll concentrations in both understory species. Canopy cover of P. abies also increased, decreasing light availability and leaf mass per area of V. myrtillus. Among leaves of either shrub, foliar N content did not explain variation in light-saturated CO2 exchange rates. Instead photosynthetic capacity varied with stomatal conductance possibly reflecting plant hydraulic properties and within-site variation in water availability. Moreover, likely due to increased shading under P. abies and due to water limitations in the sandy soil under P. sylvestris, individuals of the two shrubs did not increase their biomass or shift their allocation between above- and belowground parts in response to N additions. Altogether, our results indicate that the understory shrubs in these systems show little response to N additions in terms of photosynthetic physiology or growth and that changes in their performance are mostly associated with responses of the tree canopy.






Similar content being viewed by others
References
Axelsson E, Axelsson B (1986) Changes in carbon allocation patterns in spruce and pine trees following irrigation and fertilization. Tree Physiol 2:189–204
Bergh J, Linder S, Lundmark T, Elfving B (1999) The effect of water and nutrient availability on the productivity of Norway spruce in northern and southern Sweden. For Ecol Manage 119:51–62
Bhatti J, Jassal R, Black T (2012) Decarbonization of the atmosphere: role of the boreal forest under changing climate. Springer, the Netherlands
Bubier JL, Smith R, Juutinen S, Moore TR, Minocha R, Long S, Minocha S (2011) Effects of nutrient addition on leaf chemistry, morphology, and photosynthetic capacity of three bog shrubs. Oecologia 167:355–368
Cajander AK (1949) Forest types and their significance. Suomen Metsätieteellinen Seura, Helsinki
Dentener F, Drevet J, Lamarque JF, Bey I, Eickhout B, Fiore AM, Hauglustaine D, Horowitz LW, Krol M, Kulshrestha UC, Lawrence M, Galy-Lacaux C, Rast S, Shindell D, Stevenson D, Van Noije T, Atherton C, Bell N, Bergman D, Butler T, Cofala J, Collins B, Doherty R, Ellingsen K, Galloway J, Gauss M, Montanaro V, Müller JF, Pitari G, Rodriguez J, Sanderson M, Solmon F, Strahan S, Schultz M, Sudo K, Szopa S, Wild O (2006) Nitrogen and sulfur deposition on regional and global scales: a multimodel evaluation. Global Biogeochem Cycles 20:GB4003
Esseen P, Ehnström B, Ericson L, Sjöberg K (1997) Boreal forests. Ecol Bull 46:16–47
Evans JR (1989) Photosynthesis and nitrogen relationships in leaves of C-3 plants. Oecologia 78:9–19
Evans JR, Poorter H (2001) Photosynthetic acclimation of plants to growth irradiance: the relative importance of specific leaf area and nitrogen partitioning in maximizing carbon gain. Plant Cell Environ 24:755–767
Ewers BE, Oren R, Sperry JS (2000) Influence of nutrient versus water supply on hydraulic architecture and water balance in Pinus taeda. Plant Cell Environ 23:1055–1066
Field C, Mooney H (1986) The Photosynthesis-Nitrogen Relationship in Wild Plants. In: Givnish TJ (ed) The economy of plant form and function. Cambridge University Press, Cambridge, pp 25–55
Flower-Ellis, JGK (1971) Age structure and dynamics in stands of bilberry (Vaccinium myrtillus L.). Ph.D. Thesis, Department of Forest Ecology and Forest Soils. Royal College of Forestry Research Notes, Stockholm
Gebauer G, Melzer A, Rehder H (1984) Nitrate content and nitrate reductase-activity in Rumex obtusifolius L.1. Differences in organs and diurnal changes. Oecologia 63:136–142
Gerdol R, Iacumin P, Marchesini R, Bragazza L (2000) Water- and nutrient-use efficiency of a deciduous species, Vaccinium myrtillus, and an evergreen species, V. vitis-idaea, in a subalpine dwarf shrub heath in the southern Alps, Italy. Oikos 88:19–32
Givnish TJ (2002) Adaptive significance of evergreen vs. deciduous leaves: solving the triple paradox. Silv Fenn 36:703–743
Grelet GA, Alexander IJ, Proe MF, Frossard JS, Millard P (2001) Leaf habit influences nitrogen remobilization in Vaccinium species. J Exp Bot 52:993–1002
Gundale MJ, Deluca TH, Nordin A (2011) Bryophytes attenuate anthropogenic nitrogen inputs in boreal forests. Global Change Biol 17:2743–2753
Gundale MJ, From F, Bach LH, Nordin A (2013) Anthropogenic nitrogen deposition in boreal forests has a minor impact on the global carbon cycle. Global Change Biol 20:276–286
Hikosaka K, Terashima I (1995) A model of the acclimation of photosynthesis in the leaves of C-3 plants to sun and shade with respect to nitrogen use. Plant Cell Environ 18:605–618
Ingestad T (1973) Mineral nutrient requirements of Vaccinium vitis-idaea and Vaccinium myrtillus. Physiol Plant 29:239–246
Ishida TA, Nordin A (2010) No evidence that nitrogen enrichment affect fungal communities of Vaccinium roots in two contrasting boreal forest types. Soil Biol Biochem 42:234–243
Jacobson S, Pettersson F (2010) An assessment of different fertilization regimes in three boreal coniferous stands. Silv Fenn 44:815–827
Karlsson PS (1985) Effects of water and mineral nutrient supply on a deciduous and evergreen shrub: Vaccinium uliginosum L. and V. vitis-idaea. Holarct Ecol 8:1–8
Kolari P, Pumpanen J, Kulmala L, Ilvesniemi H, Nikinmaa E, Gronholm T, Hari P (2006) Forest floor vegetation plays an important role in photosynthetic production of boreal forests. For Ecol Manage 221:241–248
Kulmala L, Pumpanen J, Kolari P, Muukkonen P, Hari P, Vesala T (2011) Photosynthetic production of ground vegetation in different-aged Scots pine (Pinus sylvestris) forests. Can J For Res 41:2020–2030
Lawlor DW, Boyle FA, Young AT, Keys AJ, Kendall AC (1987) Nitrate nutrition and temperature effects on wheat—photosynthesis and photorespiration of leaves. J Exp Bot 38:393–408
Levula J, Ilvesniemi H, Westman CJ (2003) Relation between soil properties and tree species composition in a Scots pine-Norway spruce stand in southern Finland. Silv Fenn 37:205–218
Litton CM, Raich JW, Ryan MG (2007) Carbon allocation in forest ecosystems. Global Change Biol 13:2089–2109
Mäkipää R (1995) Effect of nitrogen input on carbon accumulation of boreal forest soils and ground vegetation. For Ecol Manage 79:217–226
Mäkipää R (1999) Response patterns of Vaccinium myrtillus and V. vitis-idaea along nutrient gradients in boreal forest. J Veg Sci 10:17–26
Mälkönen E, Derome J, Kukkola M (1990) Effects of nitrogen inputs on forest ecosystems estimation based on long-term fertilization experiments. In: Kauppi P, Anttila P, Kenttämies K (eds) Acidification in Finland. Springer, Berlin, pp 325–347
Manninen OH, Stark S, Kytoviita MM, Lampinen L, Tolvanen A (2009) Understory plant and soil responses to disturbance and increased nitrogen in boreal forests. J Veg Sci 20:311–322
Misson L, Baldocchi DD, Black TA, Blanken PD, Brunet Y, Yuste JC, Dorsey JR, Falk M, Granier A, Irvine MR, Jarosz N, Lamaud E, Launiainen S, Law BE, Longdoz B, Loustau D, Mckay M, Paw KT, Vesala T, Vickers D, Wilson KB, Goldstein AH (2007) Partitioning forest carbon fluxes with overstory and understory eddy-covariance measurements: a synthesis based on FLUXNET data. Agric For Meteorol 144:14–31
Montgomery RA, Givnish TJ (2008) Adaptive radiation of photosynthetic physiology in the Hawaiian lobeliads: dynamic photosynthetic responses. Oecologia 155:455–467
Moren AS, Lindroth A (2000) CO2 exchange at the floor of a boreal forest. Agric For Meteorol 101:1–14
Näsholm T, Ericsson A (1990) Seasonal changes in amino-acids, protein and total nitrogen in needles of fertilized Scots pine trees. Tree Physiol 6:267–281
Nohrstedt HO (2001) Response of coniferous forest ecosystems on mineral soils to nutrient additions: a review of Swedish experiences. Scand J For Res 16:555–573
Nordin A, Näsholm T (1997) Nitrogen storage forms in nine boreal understory plant species. Oecologia 110:487–492
Nordin A, Strengbom J, Witzell J, Nasholm T, Ericson L (2005) Nitrogen deposition and the biodiversity of boreal forests: implications for the nitrogen critical load. Ambio 34:20–24
Nordin A, Strengbom J, Forsum A, Ericson L (2009) Complex biotic interactions drive long-term vegetation change in a nitrogen enriched boreal forest. Ecosystems 12:1204–1211
O’Connell KEB, Gower ST, Norman JM (2003) Net ecosystem production of two contrasting boreal black spruce forest communities. Ecosystems 6:248–260
Oren R, Schulze ED, Matyssek R, Zimmermann R (1986) Estimating photosynthetic rate and annual carbon gain in conifers from specific leaf weight and leaf biomass. Oecologia 70:187–193
Palmqvist K, Sundberg B (2001) Characterizing photosynthesis and respiration in freshly isolated or cultured lichen photobionts. In: Kranner I, Beckett R, Varma A (eds) Protocols in lichenology. Culturing, biochemistry, ecophysiology and use in biomonitoring. Springer, Berlin, pp 152–181
Palmroth S, Berninger F, Nikinmaa E, Lloyd J, Pulkkinen P, Hari P (1999) Structural adaptation rather than water conservation was observed in Scots pine over a range of wet to dry climates. Oecologia 121:302–309
Palmroth S, Katul GG, Maier CA, Ward E, Manzoni S, Vico G (2013) On the complementary relationship between marginal nitrogen and water-use efficiencies among Pinus taeda leaves grown under ambient and CO2-enriched environments. Ann Bot Lond 111:467–477
Pasquini SC, Santiago LS (2012) Nutrients limit photosynthesis in seedlings of a lowland tropical forest tree species. Oecologia 168:311–319
Phil-Karlsson G, Akselsson C, Hellsten S, Karlsson PE, Malm G (2009) Övervakning av luftföroreningar i norra Sverige—mätningar och modellering. Svenska Miljöinstitutet, Stockholm
Poorter H, Niklas KJ, Reich PB, Oleksyn J, Poot P, Mommer L (2012) Biomass allocation to leaves, stems and roots: meta-analyses of interspecific variation and environmental control. New Phytol 193:30–50
Posada JM, Lechowicz MJ, Kitajima K (2009) Optimal photosynthetic use of light by tropical tree crowns achieved by adjustment of individual leaf angles and nitrogen content. Ann Bot 103:795–805
Riddoch I, Lehto T, Grace J (1991) Photosynthesis of tropical tree seedlings in relation to light and nutrient supply. New Phytol 119:137–147
Ritchie JC (1955) Biological flora of the British Isles Vaccinium vitis-idaea L. J Ecol 43:701–708
Ritchie JC (1956) Biological flora of the British Isles Vaccinium myrtillus L. J Ecol 44:291–299
Sabine CL, Heinmann M, Artaxo P, Bakker DCE, Chen CTA, Field CB, Gruber N, Le Quere C, Prinn RG, Richey JE, Lankao PR, Sathaye JA, Valentini R (2004) The global cycle SCOPE 62. In: Field CB, Raupach MR (eds) Current status and past trends on the global carbon cycle. Island Press, Washington, DC
Söyrinki N, Salmela R, Suvanto J (1977) Oulangan kansallispuiston metsä- ja suokasvillisuus. Summary: the forest and mire vegetation of the Oulanka national park, northern Finland. Acta For Fenn 154:1–150
Stenberg P (1998) Implications of shoot structure on the rate of photosynthesis at different levels in a coniferous canopy using a model incorporating grouping and penumbra. Funct Ecol 12:82–91
Strengbom J, Nordin A, Nasholm T, Ericson L (2002) Parasitic fungus mediates changes in nitrogen-exposed boreal forest vegetation. J Ecol 90:61–67
Tamm CO (1991) Nitrogen in terrestrial ecosystems: questions of productivity, vegetational changes, and ecosystem stability. Springer, Berlin
Thompson WA, Huang LK, Kriedemann PE (1992) Photosynthetic response to light and nutrients in sun-tolerant and shade-tolerant rain-forest trees. II. Leaf gas-exchange and component processes of photosynthesis. Aust J Plant Physiol 19:19–42
Thornley JHM (2002) Instantaneous canopy photosynthesis: analytical expressions for sun and shade leaves based on exponential light decay down the canopy and an acclimated non-rectangular hyperbola for leaf photosynthesis. Ann Bot 89:451–458
Vapaavuori EM, Vuorinen AH, Aphalo PJ, Smolander H (1995) Relationship between net photosynthesis and nitrogen in Scots pine—seasonal-variation in seedlings and shoots. Plant Soil 168:263–270
Vitousek P, Howarth R (1991) Nitrogen limitation on land and in the sea—how can it occur. Biogeochemistry 13:87–115
Vuokko S (1984) Puolukka. In: Vuokko S (ed) Suomen Luonto Kasvit. Weilin Göös, Helsinki, pp 168–169
Warren CR, Dreyer E, Adams MA (2003) Photosynthesis–Rubisco relationships in foliage of Pinus sylvestris in response to nitrogen supply and the proposed role of Rubisco and amino acids as nitrogen stores. Trees Struct Funct 17:359–366
Widen B (2002) Seasonal variation in forest-floor CO2 exchange in a Swedish coniferous forest. Agric For Meteorol 111:283–297
Zhang SS, Hennessey TC, Heinemann RA (1997) Acclimation of loblolly pine (Pinus taeda) foliage to light intensity as related to leaf nitrogen availability. Can J For Res 27:1032–1040
Acknowledgments
The authors wish to thank Ann Sehlstedt and Otilia Johansson for their assistance with field and lab work. This project was part of a joint research program (Sustainable Management of Carbon and Nitrogen in Future Forests) founded by FORMAS between the Swedish University of Agricultural Sciences and Umeå University. S. P. also acknowledges the support of the US Department of Energy through the Office of Biological and Environmental Research Terrestrial Carbon Processes program (DE-SC0006967, DE-0006700).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Hermann Heilmeier.
Rights and permissions
About this article
Cite this article
Palmroth, S., Holm Bach, L., Nordin, A. et al. Nitrogen-addition effects on leaf traits and photosynthetic carbon gain of boreal forest understory shrubs. Oecologia 175, 457–470 (2014). https://doi.org/10.1007/s00442-014-2923-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-014-2923-9