Abstract
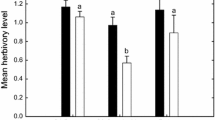
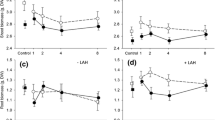
Research on trophic cascades in terrestrial ecosystems has only recently revealed that root-associated organisms interact with organisms living on aboveground plant parts. Arbuscular mycorrhizal (AM) symbiosis is a ubiquitous phenomenon, yet studies on its effect on aboveground natural enemies of herbivores are scarce and mainly deal with plant-mediated rather than herbivore-mediated interactions. Here, we studied herbivore-mediated effects of AM symbiosis on an acarine predator. We measured life history characteristics and population growth rates of Phytoseiulus persimilis preying on two-spotted spider mites, Tetranychus urticae, which were feeding on bean plants colonized or not colonized by the AM fungus Glomus mosseae. All major life history characteristics of P. persimilis, foremost oviposition rate, minimum prey requirements needed to reach adulthood, and developmental time, were positively affected by AM colonization of the host plant of their prey, together resulting in enhanced population growth rates of the predators. Hence, we hypothesize that a bottom-up trophic cascade may counteract the apparent negative effects of mycorrhizal symbiosis when promoting herbivory by promoting the predation of herbivores due to improved prey quality. We argue that this pathway may be involved in stabilizing plant–mycorrhizal symbiosis in ecosystems over time.



Similar content being viewed by others
References
Bennett AE, Bever JD (2007) Mycorrhizal species differentially alter plant growth and response to herbivory. Ecology 88:210–218
Bennett AE, Alers-Garcia J, Bever JD (2006) Three-way interactions among mutualistic mycorrhizal fungi, plants, and plant enemies: hypotheses and synthesis. Am Nat 167:141–152
Bernardo J (1996) Maternal effects in animal ecology. Am Zool 36:83–105
Bezemer TM, De Deyn GB, Bossinga TM, van Dam NM, Harvey JA, van der Putten WH (2005) Soil community composition drives aboveground plant–herbivore–parasitoid interactions. Ecol Lett 8:652–661
Bjornson S, Raworth DA (2003) Effects of plant nutrition on the expression of abdominal discoloration in Phytoseiulus persimilis (Acari: Phytoseiidae). Can Entomol 135:129–138
Blackwood JS, Schausberger P, Croft BA (2001) Prey-stage preference in generalist and specialist phytoseiid mites (Acari: Phytoseiidae) when offered Tetranychus urticae (Acari: Tetranychidae) eggs and larvae. Environ Entomol 30:1103–1111
Blossey B, Hunt-Joshi TR (2003) Belowground herbivory by insects: influence on plants and aboveground herbivores. Annu Rev Entomol 48:521–547
Boersma M, Elser JJ (2006) Too much of a good thing: on stoichiometrically balanced diets and maximal growth. Ecology 87:1325–1330
Borowicz VA (1997) A fungal root symbiont modifies plant resistance to an insect herbivore. Oecologia 112:534–542
Chang GC, Eigenbrode SD (2004) Delineating the effects of a plant trait on interactions among associated insects. Oecologia 139:123–130
Dicke M, van Loon JJA (2000) Multitrophic effects of herbivore-induced plant volatiles in an evolutionary context. Entomol Exp Appl 97:237–249
Escudero LA, Ferragut F (2005) Life-history of predatory mites Neoseiulus californicus and Phytoseiulus persimilis (Acari: Phytoseiidae) on four spider mite species as prey, with special reference to Tetranychus evansi (Acari: Tetranychidae). Biol Control 32:378–384
Forkner RE, Hunter MD (2000) What goes up must come down? Nutrient addition and predation pressure on oak herbivores. Ecology 81:1588–1600
Gange AC (2007) Insect–mycorrhizal interactions: patterns, processes and consequences. In: Ohgushi T, Craig P, Price PW (eds) Ecological communities: plant mediation in indirect interaction webs. Cambridge University Press, Cambridge, pp 134–144
Gange AC, Brown VK, Aplin DM (2003) Multitrophic links between arbuscular mycorrhizal fungi and insect parasitoids. Ecol Lett 6:1051–1055
Gehring CA, Whitham TG (2002) Mycorrhizae–herbivore interactions: population and community consequences. In: van der Heijden MGA, Sanders IR (eds) Mycorrhizal ecology, vol 157. Springer, Berlin, pp 295–320
Guerrieri E, Lingua G, Digilio MC, Massa N, Berta G (2004) Do interactions between plant roots and the rhizosphere affect parasitoid behaviour? Ecol Entomol 29:753–756
Hempel S, Stein C, Unsicker S, Renker C, Auge H, Weisser W, Buscot F (2009) Specific bottom-up effects of arbuscular mycorrhizal fungi across a plant–herbivore–parasitoid system. Oecologia 160:267–277
Hoffmann D, Vierheilig H, Riegler P, Schausberger P (2009) Arbuscular mycorrhizal symbiosis increases host plant acceptance and population growth rates of the two-spotted spider mite Tetranychus urticae. Oecologia 158:663–671
Hoffmann D, Vierheilig H, Schausberger P (2010) Arbuscular mycorrhiza enhances preference of ovipositing predatory mites for direct prey-related cues. Physiol Entomol. doi:10.1111/j.1365-3032.2010.00751.x
Hunter MD, Price PW (1992) Playing chutes and ladders—heterogeneity and the relative roles of bottom-up and top-down forces in natural communities. Ecology 73:724–732
Kagata H, Ohgushi T (2006) Bottom-up trophic cascades and material transfer in terrestrial food webs. Ecol Res 21:26–34
Koller M, Knapp M, Schausberger P (2007) Direct and indirect adverse effects of tomato on the predatory mite Neoseiulus californicus feeding on the spider mite Tetranychus evansi. Entomol Exp Appl 125:297–305
Kula AAR, Hartnett DC, Wilson GWT (2005) Effects of mycorrhizal symbiosis on tallgrass prairie plant–herbivore interactions. Ecol Lett 8:61–69
Legrand A, Barbosa P (2003) Plant morphological complexity impacts foraging efficiency of adult Coccinella septempunctata L. (Coleoptera: Coccinellidae). Environ Entomol 32:1219–1226
Maia ADN, Luiz AJB, Campanhola C (2000) Statistical inference on associated fertility life table parameters using jackknife technique: computational aspects. J Econ Entomol 93:511–518
Masters GJ, Brown VK, Gange AC (1993) Plant mediated interactions between aboveground and belowground insect herbivores. Oikos 66:148–151
Mayland H, Margolies DC, Charlton RE (2000) Local and distant prey-related cues influence when an acarine predator leaves a prey patch. Entomol Exp Appl 96:245–252
McMurtry JA, Croft BA (1997) Life-styles of phytoseiid mites and their role in biological control. Annu Rev Entomol 42:291–321
McMurtry JA, Rodriguez JG (1987) Nutritional ecology of phytoseiid mites. In: Slansky F, Rodriguez J (eds) Nutritional ecology of insects, mites and spiders. Wiley, New York, pp 609–644
McMurtry JA, Scriven GT (1965) Insectary production of phytoseiid mites. J Econ Entomol 58:282–283
Nagelkerke CJ, Sabelis MW (1998) Precise control of sex allocation in pseudo-arrhenotokous phytoseiid mites. J Evol Biol 11:649–684
Overmeer WPJ (1985) Alternative prey and other food resources. In: Helle W, Sabelis MW (eds) Spider mites: their biology, natural enemies and control, vol 1B. Elsevier, Amsterdam, pp 131–137
Read DJ (1998) Mycorrhiza—the state of the art. In: Varma A, Hock B (eds) Mycorrhiza. Springer, Berlin, pp 3–34
Sabelis MW (1985) Capacity for population increase. In: Helle W, Sabelis MW (eds) Spider mites: their biology, natural enemies and control, vol 1B. Elsevier, Amsterdam, pp 35–40
Sabelis MW, Dicke M (1985) Long-range dispersal and searching behaviour. In: Helle W, Sabelis MW (eds) Spider mites: their biology, natural enemies and control, vol 1B. Elsevier, Amsterdam, pp 141–160
Schausberger P (1997) Inter- and intraspecific predation on immatures by adult females in Euseius finlandicus, Typhlodromus pyri and Kampimodromus aberrans (Acari: Phytoseiidae). Exp Appl Acarol 21:131–150
Schausberger P, Hoffmann D (2008) Maternal manipulation of hatching asynchrony limits sibling cannibalism in the predatory mite Phytoseiulus persimilis. J Anim Ecol 77:1109–1114
Schmidt G (1976) Influence of traces left behind by its prey on searching behavior and searching success of Phytoseiulus persimilis A. & H. (Acarina-Phytoseiidae). J Appl Entomol 82:216–218
Schmitz OJ, Hamback PA, Beckerman AP (2000) Trophic cascades in terrestrial systems: a review of the effects of carnivore removals on plants. Am Nat 155:141–153
Smith SE, Read DJ (2008) Mycorrhizal symbiosis, 3rd edn. Elsevier, Amsterdam
Soler R, Bezemer TM, van der Putten WH, Vet LEM, Harvey JA (2005) Root herbivore effects on above-ground herbivore, parasitoid and hyperparasitoid performance via changes in plant quality. J Anim Ecol 74:1121–1130
Teder T, Tammaru T (2002) Cascading effects of variation in plant vigour on the relative performance of insect herbivores and their parasitoids. Ecol Entomol 27:94–104
Toyoshima S, Amano H (1998) Effect of prey density on sex ratio of two predacious mites, Phytoseiulus persimilis and Amblyseius womersleyi (Acari: Phytoseiidae). Exp Appl Acarol 22:709–723
Turlings TCJ, Guouinguené S, Degen T, Fritzsche-Hoballah ME (2002) The chemical ecology of plant–caterpillar–parasitoid interactions. In: Tscharntke T, Hawkins BA (eds) Multitrophic level interactions. Cambridge University Press, Cambridge, pp 148–173
van Dam NM, Harvey JA, Wäckers FL, Bezemer TM, van der Putten WH, Vet LEM (2003) Interactions between aboveground and belowground induced responses against phytophages. Basic Appl Ecol 4:63–77
van der Putten WH, Vet LEM, Harvey JA, Wäckers FL (2001) Linking above- and belowground multitrophic interactions of plants, herbivores, pathogens, and their antagonists. Trends Ecol Evol 16:547–554
Vanas V, Enigl M, Walzer A, Schausberger P (2006) The predatory mite Phytoseiulus persimilis adjusts patch-leaving to own and progeny prey needs. Exp Appl Acarol 39:1–11
Vierheilig H, Coughlan AP, Wyss U, Piche Y (1998) Ink and vinegar, a simple staining technique for arbuscular-mycorrhizal fungi. Appl Environ Microbiol 64:5004–5007
Vierheilig H, Bago B, Lerat S, Piche Y (2002) Shoot-produced, light-dependent factors are partially involved in the expression of the arbuscular mycorrhizal (AM) status of AM host and non-host plants. J Plant Nutr Soil Sci 165:21–25
Walker M, Hartley SE, Jones TH (2008) The relative importance of resources and natural enemies in determining herbivore abundance: thistles, tephritids and parasitoids. J Anim Ecol 77:1063–1071
Walzer A, Schausberger P (1999) Cannibalism and interspecific predation in the phytoseiid mites Phytoseiulus persimilis and Neoseiulus californicus: predation rates and effects on reproduction and juvenile development. Biocontrol 43:457–468
Walzer A, Paulus HF, Schausberger P (2004) Ontogenetic shifts in intraguild predation on thrips by phytoseiid mites: the relevance of body size and diet specialization. B Entomol Res 94:577–584
Wardle DA, Bardgett RD, Klironomos JN, Setala H, van der Putten WH, Wall DH (2004) Ecological linkages between aboveground and belowground biota. Science 304:1629–1633
Wurst S, Jones TH (2003) Indirect effects of earthworms (Aporrectodea caliginosa) on an above-ground tritrophic interaction. Pedobiologia 47:91–97
Acknowledgments
We thank Andreas Walzer and Stefan Peneder for comments on an earlier version of the manuscript. Daniela Hoffmann received a DOC-FFORTE fellowship of the Austrian Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Stefan Scheu.
Rights and permissions
About this article
Cite this article
Hoffmann, D., Vierheilig, H. & Schausberger, P. Mycorrhiza-induced trophic cascade enhances fitness and population growth of an acarine predator. Oecologia 166, 141–149 (2011). https://doi.org/10.1007/s00442-010-1821-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-010-1821-z