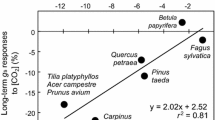
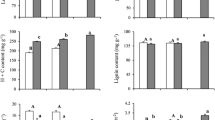
Abstract
Photosynthetic capacity is known to vary considerably among species. Its physiological cause and ecological significance have been one of the most fundamental questions in plant ecophysiology. We studied the contents of Rubisco (a key enzyme of photosynthesis) and cell walls in leaves of 26 species with a large variation in photosynthetic rates. We focused on photosynthetic nitrogen-use efficiency (PNUE, photosynthetic rate per nitrogen), which can be expressed as the product of Rubisco-use efficiency (RBUE, photosynthetic rate per Rubisco) and Rubisco nitrogen fraction (RNF, Rubisco nitrogen per total leaf nitrogen). RBUE accounted for 70% of the interspecific variation in PNUE. The variation in RBUE was ascribed partly to stomatal conductance, and other factors such as mesophyll conductance and Rubisco kinetics might also be involved. RNF was also significantly related to PNUE but the correlation was relatively weak. Cell wall nitrogen fraction (WNF, cell wall nitrogen per total leaf nitrogen) increased with increasing leaf mass per area, but there was no correlation between RNF and WNF. These results suggest that nitrogen allocation to cell walls does not explain the variation in PNUE. The difference in PNUE was not caused by a sole factor that was markedly different among species but by several factors each of which was slightly disadvantageous in low PNUE species.




Similar content being viewed by others
Abbreviations
- Ci/Ca:
-
Ratio of CO2 concentration in intercellular spaces to that in air
- LLS:
-
Leaf life span
- LMA:
-
Leaf mass per area
- N :
-
Leaf nitrogen
- R :
-
Rubisco (ribulose-1,5-bisphosphate carboxylase/oxygenase)
- RNF:
-
Rubisco nitrogen fraction
- P :
-
Photosynthetic rate
- PNUE:
-
Photosynthetic nitrogen-use efficiency
- RBUE:
-
Rubisco-use efficiency
- WM:
-
Cell wall mass
- WN:
-
Cell wall nitrogen
- WNF:
-
Cell wall nitrogen fraction
- Subscripted “area”:
-
Per unit leaf area
- Subscripted “mass”:
-
Per unit mass
References
Coley PD (1983) Herbivory and defensive characteristics of tree species in a low land tropical forest. Ecol Monogr 53:209–233
Evans JR (1989) Photosynthesis and nitrogen relationships in leaves of C3 plants. Oecologia 78:9–19
Evans JR, Seemann JR (1989) The allocation of protein nitrogen in the photosynthetic apparatus: costs, consequences and control. In: Crigs WR (ed) Photosynthesis. Liss, New York, pp 183–205
Falster D, Warton D, Wright I (2006) Standardised major axis tests and routines. Version 2. http://www.bio.mq.edu.au/ecology/SMATR
Farquhar GD, von Caemmerer S, Berry JA (1980) A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 149:78–90
Felsenstein J (1985) Phylogenies and the comparative method. Am Nat 125:1–15
Feng YL (2008) Nitrogen allocation and partitioning in invasive and native Eupatorium species. Physiol Plant 132:350–358
Feng YL, Auge H, Ebeling SK (2007) Invasive Buddleja davidii allocates more nitrogen to its photosynthetic machinery than five native woody species. Oecologia 153:501–510
Field C, Mooney HA (1986) The photosynthesis–nitrogen relationship in wild plants. In: Givnish TJ (ed) On the economy of plant form and function. Cambridge University Press, Cambridge, pp 25–55
Field C, Merino J, Mooney HA (1983) Compromises between water-use efficiency and nitrogen-use efficiency in five species of California evergreens. Oecologia 60:384–389
Flexas J, Ribas-Carbó M, Diaz-Espejo A, Galmés J, Medrano H (2008) Mesophyll conductance to CO2: current knowledge and future prospects. Plant Cell Environ 31:602–621
Fry SC (1988) The growing plant cell wall. Longman, Harlow
Galmés J, Flexas J, Keys AJ, Cifre J, Mitchell RAC, Madgwick PJ, Haslam RP, Medrano H, Parry MAJ (2005) Rubisco specificity factor tends to be larger in plant species from drier habitats and in species with persistent leaves. Plant Cell Environ 28:571–579
Gleadow RM, Foley WJ, Woodrow IE (1998) Enhanced CO2 alters the relationship between photosynthesis and defence in cyanogenic Eucalyptus cladocalyx F. Muell. Plant Cell Environ 21:12–22
Harrison MT, Edwards EJ, Farquhar GD, Nicotra AB, Evans JR (2009) Nitrogen in cell walls of sclerophyllous leaves accounts for little of the variation in photosynthetic nitrogen use efficiency. Plant Cell Environ 32:259–270
Hasebe M, Ando T, Iwatsuki K (1998) Intrageneric relationships of maple trees based on the chloroplast DNA restriction fragment length polymorphisms. J Plant Res 111:441–451
Hikosaka K (2004) Interspecific difference in the photosynthesis–nitrogen relationship: patterns, physiological causes, and ecological importance. J Plant Res 117:481–494
Hikosaka K (2005) Leaf canopy as a dynamic system: ecophysiology and optimality in leaf turnover. Ann Bot 95:521–533
Hikosaka K, Hirose T (2000) Photosynthetic nitrogen-use efficiency in species coexisting in a warm-temperate evergreen forest. Tree Physiol 20:1249–1254
Hikosaka K, Hanba YT, Hirose T, Terashima I (1998) Photosynthetic nitrogen-use efficiency in woody and herbaceous plants. Funct Ecol 12:896–905
Kogami H, Hanba YT, Kibe T, Terashima I, Masuzawa T (2001) CO2 transfer conductance, leaf structure and carbon isotope composition of Polygonum cuspidatum leaves from low and high altitudes. Plant Cell Environ 24:529–538
Lamb Frye AS, Kron KA (2003) rbcL phylogeny and character evolution in Polygonaceae. Syst Bot 28:326–332
Lloyd J, Syvertsen JP, Kriedemann PE, Farquhar GD (1992) Low conductances for CO2 diffusion from stomata to the sites of carboxylation in leaves of woody species. Plant Cell Environ 15:873–899
Martins EP (2004) COMPARE Version 4.6b. http://compare.bio.indiana.edu/~martinsl/compare/
Niinemets Ü, Tenhunen JD (1997) A model separating leaf structural and physiological effects on carbon gain along light gradients for the shade-tolerant species Acer saccharum. Plant Cell Environ 20:845–866
Ohyama M, Baba K, Itoh T, Shiraishi S (1999) Polymorphism analysis of Fagaceae and DNA-based identification of Fagus species grown in Japan based on the rbcL gene. J Wood Sci 45:183–187
Onoda Y, Hikosaka K, Hirose T (2004) Allocation of nitrogen to cell walls decreases photosynthetic nitrogen-use efficiency. Funct Ecol 18:419–425
Onoda Y, Schieving F, Anten NPR (2008) Effects of light and nutrient availability on leaf mechanical properties of Plantago major: a conceptual approach. Ann Bot 101:727–736
Pons TL, Westbeek MHK (2004) Analysis of differences in photosynthetic nitrogen-use efficiency between four contrasting species. Physiol Plant 122:68–78
Pons TL, van der Werf A, Lambers H (1994) Photosynthetic nitrogen use efficiency of inherently low- and fast-growing species: possible explanations for observed differences. In: Roy J, Garnier E (eds) A whole plant perspective on carbon–nitrogen interactions. SPB, The Hague, pp 61–77
Poorter H, Evans JR (1998) Photosynthetic nitrogen-use efficiency of species that differ inherently in specific area. Oecologia 116:26–37
Poorter H, Farquhar GD (1994) Transpiration, intercellular carbon dioxide concentration and carbon-isotope discrimination of 24 wild species differing in relative growth rate. Aust J Plant Physiol 21:507–516
Reich PB, Walters MB, Ellsworth DS (1991) Leaf lifespan as a determinant of leaf structure and function among 23 Amazonian tree species. Oecologia 86:16–24
Reich PB, Walters MB, Ellsworth DS (1992) Leaf life-span in relation to leaf, plant, and stand characteristics among diverse ecosystems. Ecol Monogr 62:365–392
Reich PB, Walters MB, Ellsworth DS (1997) From tropics to tundra: Global convergence in plant functioning. Proc Nat Acad Sci USA 94:13730–13734
Stevens PF (2008) Angiosperm phylogeny website. Version 9. http://www.mobot.org/MOBOT/research/APweb/
Takashima T, Hikosaka K, Hirose T (2004) Photosynthesis or persistence: nitrogen allocation in leaves of evergreen and deciduous Quercus species. Plant Cell Environ 27:1047–1054
Terashima I, Hanba YT, Tazoe Y, Vyas P, Yano S (2006) Irradiance and phenotype: comparative eco-development of sun and shade leaves in relation to photosynthetic CO2 diffusion. J Exp Bot 57:343–354
von Caemmerer S, Evans JR, Hudson GS, Andrews TJ (1994) The kinetics of ribulose-1,5-bisphosphate carboxylase/oxygenase in vivo inferred from measurements of photosynthesis in leaves of transgenic tobacco. Planta 195:88–97
Warren CR (2008) Stand aside stomata, another actor deserves centre stage: the forgotten role of the internal conductance to CO2 transfer. J Exp Bot 59:1475–1487
Warren CR, Adams MA (2004a) Evergreen trees do not maximize instantaneous photosynthesis. Trends Plant Sci 9:270–274
Warren CR, Adams MA (2004b) What determines rates of photosynthesis per unit nitrogen in Eucalyptus seedlings? Funct Plant Biol 31:1169–1178
Warren CR, Adams MA (2006) Internal conductance does not scale with photosynthetic capacity: implications for carbon isotope discrimination and the economics of water and nitrogen use in photosynthesis. Plant Cell Environ 29:192–201
Warren CR, Dreyer E, Tausz M, Adams MA (2006) Ecotype adaptation and acclimation of leaf traits to rainfall in 29 species of 16-year-old Eucalyptus at two common gardens. Funct Ecol 20:929–940
Warton DI, Wright IJ, Falster DS, Westoby M (2006) Bivariate line-fitting methods for allometry. Biol Rev 81:259–291
Westbeek HMH, Pons TL, Cambridge ML, Atkin OK (1999) Analysis of differences in photosynthetic nitrogen use efficiency of alpine and lowland Poa species. Oecologia 120:19–26
Wright IJ, Cannon K (2001) Relationships between leaf lifespan and structural defences in a low-nutrient, sclerophyll flora. Funct Ecol 15:351–359
Wright IJ, Reich PB, Westoby B et al (2004) The worldwide leaf economics spectrum. Nature 428:821–827
Wright IJ, Reich PB, Cornelissen JHC et al (2005) Assessing the generality of global leaf trait relationships. New Phytol 166:485–496
Yoshie F (1986) Intercellular CO2 concentration and water-use efficiency of temperate plants with different life-forms and from different microhabitats. Oecologia 68:370–374
Acknowledgments
We thank Shimpei Oikawa, Yusuke Onoda, Yuko Yasumura, Onno Muller and John Evans for kind help in the field experiment and valuable suggestions. This study was supported in part by grants from the Japan Ministry of Education, Culture, Sports, Science and Technology (KAKENHI) and from the Global Environment Research Fund (F-052) from the Japan Ministry of the Environment and by the GCOE program J03 of the MEXT, Japan.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by R. Sage.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hikosaka, K., Shigeno, A. The role of Rubisco and cell walls in the interspecific variation in photosynthetic capacity. Oecologia 160, 443–451 (2009). https://doi.org/10.1007/s00442-009-1315-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-009-1315-z