Abstract
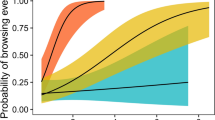
A basic idea of plant defences is that a plant should gain protection from its own defence. In addition, there is evidence that defence traits of the neighbouring plants can influence the degree of protection of an individual plant. These associational effects depend in part on the spatial scale of herbivore selectivity. A strong between-patch selectivity together with a weak within-patch selectivity leads to a situation where a palatable plant could avoid being grazed by growing in a patch with unpalatable plants, which is referred to as associational defence. Quite different associational effects will come about if the herbivore instead is unselective between patches and selective within a patch. We studied these effects in a manipulative experiment where we followed the food choice of fallow deer when they encountered two patches of overall different quality. One of the two patches consisted of pellets with low-tannin concentration in seven out of eight buckets and with high concentration in the remaining bucket. The other patch instead had seven high- and one low-tannin bucket. We performed the experiment both with individuals one at a time and with a group of 16–17 deer. We found that the deer were unselective between patches, but selective within a patch, and that the single low-tannin bucket among seven high-tannin buckets was used more than a low-tannin bucket among other low-tannin buckets. This corresponds to a situation where a palatable plant that grows among unpalatable plants is attacked more than if it was growing among its own kind, and for this effect we suggest the term neighbour contrast susceptibility, which is the opposite of associational defence. We also found that the high-tannin bucket in the less defended patch was less used than the high-tannin buckets in the other patch, which corresponds to neighbour contrast defence. The neighbour contrast susceptibility was present both for individual and group foraging, but the strength of the effect was somewhat weaker for groups due to weaker within-patch selectivity.


Similar content being viewed by others
References
Alm U, Birgersson B, Leimar O (2002) The effect of food quality and relative abundance on food choice in fallow deer. Anim Behav 64:439–445
Alm Bergvall U, Leimar O (2005) Plant secondary compounds and the frequency of food types affect food choice by mammalian herbivores. Ecology 86(9):2450–2460
Atsatt PR, O’Dowd DJ (1976) Plant defense guilds. Science 193:24–29
Augustine DJ, Mc Naughton SJ (1998) Ungulate effects on the functional species composition of plant communities: herbivore selectivity and plant tolerance. J Wildl Manage 62:1165–1183
Boissy A, Dumont B (2002) Interaction between social and feeding motivations on the grazing behaviour of herbivores: sheep more easily split into subgroups with familiar peers. Appl Anim Behav Sci 79:233–245
Brown JS, Morgan RA (1995) Effects of foraging behavior and spatial scale on diet selectivity: a test with fox squirrels. Oikos 74:122–136
Bryant JP, Reichardt PB, Clausen TP, Provenza FD, Kuropat PJ (1989) Woody plant–mammal interactions. In: Rosenthal GA, Berenbaum MR (eds) Herbivores: their interaction with secondary plant metabolites. Academic, New York, pp 343–370
Callaway RM, Kikodze D, Chiboshvili M, Khetsuriani L (2005) Unpalatable plants protect neighbors from grazing and increase plant community diversity. Ecology 86:1856–1862
Dall SRX, Giraldeau L-A, Olsson O, McNamara JM, Stephens DW (2005) Information and its use by animals in evolutionary ecology. Trends Ecol Evol 20:187–193
Distel RA, Laca EA, Griggs TC, Demment MW (1995) Patch selection by cattle: maximization of intake rate in horizontally heterogeneous pastures. Appl Anim Behav Sci 45(1–2):11–21
Freeland WJ (1991) Plant secondary metabolites: biochemical coevolution with herbivores. In: Palo RT, Robbins CT (eds) Plant defences against mammalian herbivory. CRC Press, Boca Raton, pp 61–81
Fritz H, De Garine-Wichatitsky M (1996) Foraging in a social antelope: effects of group size on foraging choices and resource perception in impala. J Anim Ecol 65:736–742
Hambäck PA, Ågren J, Ericson L (2000) Associational resistance: insect damage to purple loosestrife reduced in thickets of sweet gale. Ecology 81(7):1784–1794
Hayakawa Y (1972) Hokkiado Nogyo Shikenjo Iho
Hjältén J, Danell K, Lundberg P (1993) Herbivore avoidance by association: vole and hare utilization of woody plants. Oikos 68:125–131
Hokkanen HMT (1991) Trap cropping in pest-management. Annu Rev Entomol 36:119–138
Krebs JR, Davies NB (1999) An introduction to behavioural ecology. Blackwell Science, Oxford
Leimar O, Tuomi J (1998) Synergistic selection and graded traits. Evol Ecol 12:59–71
Mali S, Borges RM (2003) Phenolics, fibre, alkaloids, saponins, and cyanogenic glycosides in a seasonal cloud forest in India. Biochem Syst Ecol 31:1221–1246
Matthews S, Mila I, Scalbert A, Donnelly DMX (1997) Extractable and non-extractable proanthocyanidins in barks. Phytochemistry 45:405–410
Milchunas DG, Noy-Meir I (2002) Grazing refuges, external avoidance of herbivory and plant diversity. Oikos 99:113–130
Molvar EM, Bowyer RT (1994) Costs and benefits of group living in a recently social ungulate: the Alaskan Moose. J Mamm 75:621–630
Morgan RA, Brown JS, Thorson JM (1997) The effect on spatial scale on the functional response of fox squirrels. Ecology 78:1087–1097
Olff H, Vera FWM, Bokdam J, Bakker ES, Gleichman JM, de Mayer K, Smit R (1999) Shifting mosaics in grazed woodlands driven by the alternation of plant facilitation and competition. Plant Biol 1:127–137
Palo RT, Robbins CT (1991) Plant defences against mammalian herbivory. CRC Press, Boca Raton
Pfister CA, Hay ME (1988) Associational plant refuges: convergent patterns in marine and terrestrial communities result from differing mechanisms. Oecologia 77:118–129
Phillips JD, Pfieffer RK (1958) Proceedings of the 4th British Weed control Conference
Quinn GP, Keough MJ (2002) Experimental design and data analysis for biologists. Cambridge University Press, Cambridge, UK
Rhoades DF (1979) Evolution of plant chemical defense against herbivores. In: Rosenthal GA, Janzen DH (eds) Herbivores: their interaction with secondary plant metabolites. Academic, New York, pp 3–54
Städler E (1992) Behavioral responses of insects to plant secondary compounds. In: Rosenthal GA, Berenbaum MR (eds) Herbivores: their interaction with secondary plant metabolites. Academic, New York, pp 45–88
Stephen DW, Krebs JR (1986) Foraging theory. In: Krebs JR, Clutton-Brock T (eds) Monographs in behavior ecology. Princeton University Press, Princeton
Tixier H, Duncan P, Scehovic J, Yani A, Gleizes M, Lila M (1997) Food selection by European roe deer (Capreolus capreolus): effects of plant chemistry, and consequences for the nutritional value of their diets. J Zool (Lond) 242:229–245
Tuomi J, Augner M (1993) Synergistic selection of unpalatability in plants. Evolution 47:668–672
Tuomi J, Augner M, Nilsson P (1994) A dilemma of plant defenses—is it really worth killing the herbivore? J Theor Biol 170:427–430
White JA, Whitham TG (2000) Associational susceptibility of cottonwood to a box elder herbivore. Ecology 81:1795–1803
Acknowledgements
The Academy of Finland provided the funding for PR (project no. 80486). OL was supported by grants from the Swedish Research Council. Thanks are due to the personnel at Tovetorp Zoological Station (Sven Jacobson, Thomas Giegold, Adeline Öhman and Dennis Henrysson) for contributing facilities and practical help. Thanks to Torbjörn Alm for computer programming.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Hannu Ylonen
Rights and permissions
About this article
Cite this article
Alm Bergvall, U., Rautio, P., Kesti, K. et al. Associational effects of plant defences in relation to within- and between-patch food choice by a mammalian herbivore: neighbour contrast susceptibility and defence. Oecologia 147, 253–260 (2006). https://doi.org/10.1007/s00442-005-0260-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-005-0260-8