Abstract
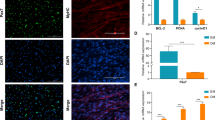
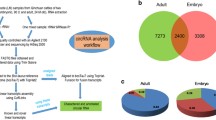
The formation of skeletal muscle is a complex process that is coordinated by many regulatory factors, such as myogenic factors and noncoding RNAs. Numerous studies have proved that circRNA is an indispensable part of muscle development. However, little is known about circRNAs in bovine myogenesis. In this study, we discovered a novel circRNA, circ2388, formed by reverse splicing of the fourth and fifth exons of the MYL1 gene. The expression of circ2388 was different between fetal and adult cattle muscle. This circRNA is 99% homologous between cattle and buffalo and is localized in the cytoplasm. Thoroughly, we proved that circ2388 had no effect on cattle and buffalo myoblast proliferation but promotes myoblast differentiation and myotube fusion. Furthermore, circ2388 in vivo stimulated skeletal muscle regeneration in mouse muscle injury model. Taken together, our findings suggest that circ2388 promotes myoblast differentiation and promotes the recovery and regeneration of damaged muscles.








Similar content being viewed by others
Data availability
The datasets used or analyzed in this study are available from the corresponding author on reasonable request.
References
Adhikari A, Kim W, Davie J (2021) Myogenin is required for assembly of the transcription machinery on muscle genes during skeletal muscle differentiation. PLoS ONE 16(1):e245618
Agarwal M, Sharma A, Kumar P, Kumar A, Bharadwaj A, Saini M et al (2020) Myosin heavy chain-embryonic regulates skeletal muscle differentiation during mammalian development. Development (Cambridge, England) 147(7)
Archacka K, Ciemerych MA, Florkowska A, Romanczuk K (2021) Non-coding rnas as regulators of myogenesis and postexercise muscle regeneration. Int J Mol Sci 22(21)
Argilés JM, Campos N, Lopez-Pedrosa JM, Rueda R, Rodriguez-Mañas L (2016) Skeletal muscle regulates metabolism via interorgan crosstalk: roles in health and disease. J Am Med Dir Assoc 17(9):789–796
Bachman JF, Klose A, Liu W, Paris ND, Blanc RS, Schmalz M et al (2018) Prepubertal skeletal muscle growth requires pax7-expressing satellite cell-derived myonuclear contribution. Development (Cambridge, England) 145(20)
Begum S, Yiu A, Stebbing J, Castellano L (2018) Novel tumour suppressive protein encoded by circular rna, circ-shprh, in glioblastomas. Oncogene 37(30):4055–4057
Buckingham M, Bajard L, Chang T, Daubas P, Hadchouel J, Meilhac S et al (2003) The formation of skeletal muscle: from somite to limb. J Anat 202(1):59–68
Capel B, Swain A, Nicolis S, Hacker A, Walter M, Koopman P et al (1993) Circular transcripts of the testis-determining gene sry in adult mouse testis. Cell 73(5):1019–1030
Chen M, Wei X, Song M, Jiang R, Huang K, Deng Y et al (2021) Circular RNA circmybpc1 promotes skeletal muscle differentiation by targeting myhc. Molecular Therapy Nucleic Acids 24:352–368
Conn SJ, Pillman KA, Toubia J, Conn VM, Salmanidis M, Phillips CA et al (2015) The RNA binding protein quaking regulates formation of circrnas. Cell 160(6):1125–1134
Du J, Zhang P, Zhao X, He J, Xu Y, Zou Q et al (2019) Microrna-351-5p mediates skeletal myogenesis by directly targeting lactamase-β and is regulated by lnc-mg. FASEB J Offic Publication Federation Am Soc Experiment Biol 33(2):1911–1926
Fu W, Wang R, Nanaei HA, Wang J, Hu D, Jiang Y (2022) Rgd v2.0: a major update of the ruminant functional and evolutionary genomics database. Nucleic Acids Res 50(D1):D1091-D1099
Hansen TB, Jensen TI, Clausen BH, Bramsen JB, Finsen B, Damgaard CK et al (2013) Natural RNA circles function as efficient microrna sponges. Nature 495(7441):384–388
Hsu MT, Coca-Prados M (1979) Electron microscopic evidence for the circular form of RNA in the cytoplasm of eukaryotic cells. Nature 280(5720):339–340
Huang K, Chen M, Zhong D, Luo X, Feng T, Song M et al (2021) Circular RNA profiling reveals an abundant circech1 that promotes myogenesis and differentiation of bovine skeletal muscle. J Agr Food Chem 69(1):592–601
Kristensen LS, Andersen MS, Stagsted LVW, Ebbesen KK, Hansen TB, Kjems J (2019) The biogenesis, biology and characterization of circular rnas. Nat Rev Genet 20(11):675–691
Legnini I, Di Timoteo G, Rossi F, Morlando M, Briganti F, Sthandier O et al (2017) Circ-znf609 is a circular RNA that can be translated and functions in myogenesis. Mol Cell 66(1):22–37
Legnini I, Morlando M, Mangiavacchi A, Fatica A, Bozzoni I (2014) A feedforward regulatory loop between hur and the long noncoding RNA linc-md1 controls early phases of myogenesis. Mol Cell 53(3):506–514
Li H, Wei X, Yang J, Dong D, Hao D, Huang Y et al (2018a) Circfgfr4 promotes differentiation of myoblasts via binding mir-107 to relieve its inhibition of wnt3a. Molecular Therapy Nucleic Acids 11:272–283
Li H, Yang J, Wei X, Song C, Dong D, Huang Y et al (2018b) Circfut10 reduces proliferation and facilitates differentiation of myoblasts by sponging mir-133a. J Cell Physiol 233(6):4643–4651
Li X, Yang L, Chen L (2018c) The biogenesis, functions, and challenges of circular rnas. Mol Cell 71(3):428–442
Li J, Su T, Zou C, Luo W, Shi G, Chen L et al (2020) Long non-coding RNA h19 regulates porcine satellite cell differentiation through mir-140-5p/sox4 and dbn1. Front Cell and Dev Biol 8
Li L, Chen Y, Nie L, Ding X, Zhang X, Zhao W et al (2019) Myod-induced circular RNA cdr1as promotes myogenic differentiation of skeletal muscle satellite cells. Biochimica et biophysica acta. Gene Regul Mech 1862(8);807–821
Mytidou C, Koutsoulidou A, Zachariou M, Prokopi M, Kapnisis K, Spyrou GM et al (2021) Age-related exosomal and endogenous expression patterns of mir-1, mir-133a, mir-133b, and mir-206 in skeletal muscles. Front Physiol 12
Nishikura K (2010) Functions and regulation of RNA editing by adar deaminases. Annu Rev Biochem 79:321–349
Pamudurti NR, Bartok O, Jens M, Ashwal-Fluss R, Stottmeister C, Ruhe L et al (2017) Translation of circrnas. Mol Cell 66(1):9–21
Park SE, Jeong JB, Oh SJ, Kim SJ, Kim H, Choi A et al (2021) Wharton's jelly-derived mesenchymal stem cells reduce fibrosis in a mouse model of duchenne muscular dystrophy by upregulating microrna 499. Biomed 9(9)
Ravenscroft G, Zaharieva IT, Bortolotti CA, Lambrughi M, Pignataro M, Borsari M et al (2018) Bi-allelic mutations in myl1 cause a severe congenital myopathy. Hum Mol Genet 27(24):4263–4272
Sanger HL, Klotz G, Riesner D, Gross HJ, Kleinschmidt AK (1976) Viroids are single-stranded covalently closed circular RNA molecules existing as highly base-paired rod-like structures. P Natl Acad Sci Usa 73(11):3852–3856
Sinha T, Panigrahi C, Das D, Chandra PA (2022) Circular RNA translation, a path to hidden proteome. Wiley Interdiscip Rev RNA 13(1)
Teplova M, Hafner M, Teplov D, Essig K, Tuschl T, Patel DJ (2013) Structure-function studies of star family quaking proteins bound to their in vivo RNA target sites. Gene Dev 27(8):928–940
Viecelli C, Aguayo D (2021) May the force and mass be with you-evidence-based contribution of mechano-biological descriptors of resistance exercise. Front Physiol 12:686119
Waldemer-Streyer RJ, Kim D, Chen J (2022) Muscle cell-derived cytokines in skeletal muscle regeneration. FEBS J
Wei X, Li H, Yang J, Hao D, Dong D, Huang Y et al (2017) Circular RNA profiling reveals an abundant circlmo7 that regulates myoblasts differentiation and survival by sponging mir-378a-3p. Cell Death Dis 8(10):e3153
Weng J, Zhang P, Yin X, Jiang B (2018) The whole transcriptome involved in denervated muscle atrophy following peripheral nerve injury. Front Mol Neurosci 11:69
Wohlwend M, Laurila P, Williams K, Romani M, Lima T, Pattawaran P et al (2021) The exercise-induced long noncoding RNA cytor promotes fast-twitch myogenesis in aging. Sci Transl Med 13(623):c7367
Ye F, Gao G, Zou Y, Zheng S, Zhang L, Ou X et al (2019) Circfbxw7 inhibits malignant progression by sponging mir-197-3p and encoding a 185-aa protein in triple-negative breast cancer. Molecular Therapy Nucleic Acids 18:88–98
Zhang M, Zhao K, Xu X, Yang Y, Yan S, Wei P et al (2018) A peptide encoded by circular form of linc-pint suppresses oncogenic transcriptional elongation in glioblastoma. Nat Commun 9(1):4475
Zhang P, Du J, Guo X, Wu S, He J, Li X et al (2021) Lncmyod promotes skeletal myogenesis and regulates skeletal muscle fiber-type composition by sponging mir-370–3p. Genes-Basel 12(4)
Funding
This work was supported by the China Postdoctoral Science Foundation (Grant No. 2019M663842), the National Natural Science Foundation of China (Grant Nos. 32102514 and U20A2051), the Guangxi Natural Science Foundation (Grant Nos. 2019GXNSFAA185056 and 2020GXNSFBA297148), and the Science and Technology Base and Talent Special in Guangxi (Grant No. AD20159062).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
The animal care and research protocol for this experiment has been approved by the Animal Care Committee of the College of Animal Science and Technology of Guangxi University.
Consent to participate
Dandan Zhong, Kongwei Huang, and Liyin Zhang were responsible for the execution of the experiment and drawing diagrams. Dandan Zhong and Kongwei Huang participated in the writing of the manuscript. Yudong Cai and Huiren Li participated in the analysis of the data. Qingyou Liu and Deshun Shi were involved in the revision of the manuscript. Hui Li and Yu Jiang participated in the experimental design and guidance. Dandan Zhong and Kongwei Huang contributed equally to this work. All authors read and approved the final manuscript.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhong, D., Huang, K., Zhang, L. et al. Circ2388 regulates myogenesis and muscle regeneration. Cell Tissue Res 393, 149–161 (2023). https://doi.org/10.1007/s00441-023-03787-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-023-03787-1