Abstract
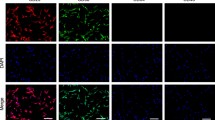
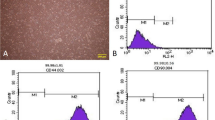
Human mesenchymal stem cells were exposed to diabetic sera for 7 days. Cell viability and apoptosis rate were detected by MTT and flow cytometry assays. The expression of key genes such as CD63, Alix, Rab27a, Rab27b, and Rab8b was monitored by real-time PCR. We also measured acetylcholinesterase activity and size and zeta potential of exosomes in the supernatant form diabetic cells and control. The cellular distribution of CD63 was shown by immunofluorescence imaging and western blotting. Any changes in the ultrastructure of cells were visualized by electron microscopy. Data showed a slight decrease in survival rate and an increased apoptosis in diabetic cells as compared to control (p < 0.05). By exposing cells to diabetic sera, a significant increase in the level of all genes CD63, Alix, Rab27a, Rab27b, and Rab8b was observed (p < 0.05). Flow cytometry analysis and immunofluorescence imaging confirmed increasing CD63 protein content upon treatment with diabetic sera (p < 0.05). We found an enhanced acetylcholinesterase activity in a diabetic condition which coincided with the increasing size of exosomes and decrease in zeta potential (p < 0.05). The fatty acid profile was not significantly affected by diabetic sera. Ultrastructural examination detected more accumulated cytoplasmic lipid vacuoles in diabetic cells.





Similar content being viewed by others
References
Akagi T, Ichiki T, Ohshima H (2016) Evaluation of zeta-potential of individual exosomes secreted from biological cells using a microcapillary electrophoresis chip. Encyclopedia of Biocolloid and Biointerface Science 2V Set 469–473
Ardestani A, Paroni F, Azizi Z, Kaur S, Khobragade V, Yuan T, Frogne T, Tao W, Oberholzer J, Pattou F (2014) MST1 is a key regulator of beta cell apoptosis and dysfunction in diabetes. Nat Med 20:385–397
Baietti MF, Zhang Z, Mortier E, Melchior A, Degeest G, Geeraerts A, Ivarsson Y, Depoortere F, Coomans C, Vermeiren E (2012) Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat Cell Biol 14:677–685
Cade WT (2008) Diabetes-related microvascular and macrovascular diseases in the physical therapy setting. Phys Ther 88:1322
Chen J, Chen S, Chen Y, Zhang C, Wang J, Zhang W, Liu G, Zhao B, Chen Y (2011) Circulating endothelial progenitor cells and cellular membrane microparticles in db/db diabetic mouse: possible implications in cerebral ischemic damage. Am J Physiol Endocrinol Metab 301:E62–E71
Chen LN, Sun Q, Liu SQ, Hu H, Lv J, Ji WJ, Wang M, Chen MX, Zhou J (2015) Erythropoietin improves glucose metabolism and pancreatic β-cell damage in experimental diabetic rats. Mol Med Rep 12:5391–5398
Choi D-S, Lee J-M, Park GW, Lim H-W, Bang JY, Kim Y-K, Kwon K-H, Kwon HJ, Kim KP, Gho YS (2007) Proteomic analysis of microvesicles derived from human colorectal cancer cells. J Proteome Res 6:4646–4655
Harding C, Heuser J, Stahl P (1983) Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J Cell Biol 97:329–339
Hassanpour M, Cheraghi O, Siavashi V, Rahbarghazi R, Nouri M (2016) A reversal of age-dependent proliferative capacity of endothelial progenitor cells from different species origin in in vitro condition. J Cardiovasc Thoracic Res 8:102–106
Hutagalung AH, Novick PJ (2011) Role of Rab GTPases in membrane traffic and cell physiology. Physiol Rev 91:119–149
Kolluru GK, Bir SC, Kevil CG (2012, 2012) Endothelial dysfunction and diabetes: effects on angiogenesis, vascular remodeling, and wound healing. Int J Vasc Med
Kowal J, Tkach M, Théry C (2014) Biogenesis and secretion of exosomes. Curr Opin Cell Biol 29:116–125
Lehmann BD, Paine MS, Brooks AM, McCubrey JA, Renegar RH, Wang R, Terrian DM (2008) Senescence-associated exosome release from human prostate cancer cells. Cancer Res 68:7864–7871
Makino N, Maeda T, Sugano M, Satoh S, Watanabe R, Abe N (2005) High serum TNF-α level in type 2 diabetic patients with microangiopathy is associated with eNOS down-regulation and apoptosis in endothelial cells. J Diabetes Complicat 19:347–355
Ostrowski M, Carmo NB, Krumeich S, Fanget I, Raposo G, Savina A, Moita CF, Schauer K, Hume AN, Freitas RP (2010) Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol 12:19–30
Pan B-T, Teng K, Wu C, Adam M, Johnstone RM (1985) Electron microscopic evidence for externalization of the transferrin receptor in vesicular form in sheep reticulocytes. J Cell Biol 101:942–948
Phinney DG, Pittenger MF (2017) Concise review: MSC-derived exosomes for cell-free therapy. Stem Cells 35:851–858
Rahbarghazi R, Nassiri SM, Ahmadi SH, Mohammadi E, Rabbani S, Araghi A, Hosseinkhani H (2014) Dynamic induction of pro-angiogenic milieu after transplantation of marrow-derived mesenchymal stem cells in experimental myocardial infarction. Int J Cardiol 173:453–466
Record M, Subra C, Silvente-Poirot S, Poirot M (2011) Exosomes as intercellular signalosomes and pharmacological effectors. Biochem Pharmacol 81:1171–1182
Rezabakhsh A, Cheraghi O, Nourazarian A, Hassanpour M, Kazemi M, Ghaderi S, Faraji E, Rahbarghazi R, Avci ÇB, Bagca BG (2017a) Type 2 diabetes inhibited human mesenchymal stem cells angiogenic response by over-activity of the autophagic pathway. J Cell Biochem 118:1518–1530
Rezabakhsh A, Nabat E, Yousefi M, Montazersaheb S, Cheraghi O, Mehdizadeh A, Fathi F, Movassaghpour AA, Maleki-Dizaji N, Rahbarghazi R (2017b) Endothelial cells’ biophysical, biochemical, and chromosomal aberrancies in high-glucose condition within the diabetic range. Cell Biochem Funct 35:83–97
Rezaie J, Ajezi S, Avci ÇB, Karimipour M, Geranmayeh MH, Nourazarian A, Sokullu E, Rezabakhsh A, Rahbarghazi R (2017) Exosomes and their application in biomedical field: difficulties and advantages. Mol Neurobiol https://doi.org/10.1007/s12035-017-0582-7
Rezaie J, Mehranjani MS, Rahbarghazi R, Shariatzadeh MA (2018) Angiogenic and restorative ability of human mesenchymal stem cells were reduced following treatment with serum from diabetes mellitus type 2 patients. J Cell Biochem 119(1):524–535
Robbins PD, Morelli AE (2014) Regulation of immune responses by extracellular vesicles. Nat Rev Immunol 14:195–208
Shabbir A, Cox A, Rodriguez-Menocal L, Salgado M, Badiavas EV (2015) Mesenchymal stem cell exosomes induce proliferation and migration of normal and chronic wound fibroblasts, and enhance angiogenesis in vitro. Stem Cells Dev 24:1635–1647
Stolzing A, Sellers D, Llewelyn O, Scutt A (2010) Diabetes induced changes in rat mesenchymal stem cells. Cells Tissues Organs 191:453–465
Stoorvogel W (2015) Resolving sorting mechanisms into exosomes. Cell Res 25:531–532
Takahashi A, Okada R, Nagao K, Kawamata Y, Hanyu A, Yoshimoto S, Takasugi M, Watanabe S, Kanemaki MT, Obuse C (2017) Exosomes maintain cellular homeostasis by excreting harmful DNA from cells. Nat Commun 8
Urbanelli L, Magini A, Buratta S, Brozzi A, Sagini K, Polchi A, Tancini B, Emiliani C (2013) Signaling pathways in exosomes biogenesis, secretion and fate. Genes 4:152–170
Van Niel G, Charrin S, Simoes S, Romao M, Rochin L, Saftig P, Marks MS, Rubinstein E, Raposo G (2011) The tetraspanin CD63 regulates ESCRT-independent and-dependent endosomal sorting during melanogenesis. Dev Cell 21:708–721
Wang L, Wang Y, Liang Y, Li J, Liu Y, Zhang J, Zhang A, Fu J, Jiang G (2014) PFOS induced lipid metabolism disturbances in BALB/c mice through inhibition of low density lipoproteins excretion. Scientific reports 4:
Wu X-m, Gao Y-b, F-q C, Zhang N (2016) Exosomes from high glucose-treated glomerular endothelial cells activate mesangial cells to promote renal fibrosis. Biol Open 5:484–491
Yan J, Tie G, Wang S, Messina KE, DiDato S, Guo S, Messina LM (2012) Type 2 diabetes restricts multipotency of mesenchymal stem cells and impairs their capacity to augment postischemic neovascularization in db/db mice. J Am Heart Assoc 1:e002238
Zeelenberg IS, Ostrowski M, Krumeich S, Bobrie A, Jancic C, Boissonnas A, Delcayre A, Le Pecq J-B, Combadière B, Amigorena S (2008) Targeting tumor antigens to secreted membrane vesicles in vivo induces efficient antitumor immune responses. Cancer Res 68:1228–1235
Acknowledgments
The authors gratefully thank the personnel of Stem Cell Research Center, Tabriz University of Medical Sciences.
Funding
This work was supported by a grant (no. TBZMED.REC.1394.928) from the Research Council, Tabriz University of Medical Sciences.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
All volunteers were asked to fill out an informed consent. All procedures performed through the current experiment, involving human participants, were in accordance with the local ethics committee of Tabriz University of Medical Sciences (Ethical code no. TBZMED.REC.1394.928) and ethical principles of Declaration of Helsinki.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Rezaie, J., Nejati, V., Khaksar, M. et al. Diabetic sera disrupted the normal exosome signaling pathway in human mesenchymal stem cells in vitro. Cell Tissue Res 374, 555–565 (2018). https://doi.org/10.1007/s00441-018-2895-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-018-2895-x