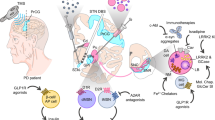
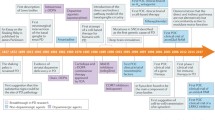
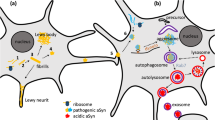
Abstract
There is currently no cure for Parkinson’s disease. The symptomatic therapeutic strategy essentially relies on dopamine replacement whose efficacy was demonstrated more than 50 years ago following the introduction of the dopamine precursor, levodopa. The spectacular antiparkinsonian effect of levodopa is, however, balanced by major limitations including the occurrence of motor complications related to its particular pharmacokinetic and pharmacodynamic properties. Other therapeutic strategies have thus been developed to overcome these problems such as the use of dopamine receptor agonists, dopamine metabolism inhibitors and non-dopaminergic drugs. Here we review the pharmacology and molecular mechanisms of dopamine replacement therapy in Parkinson’s disease, both at the presynaptic and postsynaptic levels. The perspectives in terms of novel drug development and prediction of drug response for a more personalised medicine will be discussed.





Similar content being viewed by others
References
Agid Y (2001) Levodopa. Is toxicity a myth? 1998. Neurology 57:S46–S51
Ahlskog JE (2003) Slowing Parkinson’s disease progression: recent dopamine agonist trials. Neurology 60:381–389
Ahmed MR, Bychkov E, Gurevich VV et al (2008) Altered expression and subcellular distribution of GRK subtypes in the dopamine-depleted rat basal ganglia is not normalized by l-DOPA treatment. J Neurochem 104:1622–1636
Alcacer C, Santini E, Valjent E et al (2012) Gα(olf) mutation allows parsing the role of cAMP-dependent and extracellular signal-regulated kinase-dependent signaling in L-3,4-dihydroxyphenylalanine-induced dyskinesia. J Neurosci 32:5900–5910
American Psychiatric Association (2000) Diagnostic and statistical manual of mental disorders, fourth edition, text revision (DSM-IV-TR), 4th edn. American Psychiatric Association, Arlington
Anderson E, Nutt J (2011) The long-duration response to levodopa: phenomenology, potential mechanisms and clinical implications. Parkinsonism Relat Disord 17:587–592
Andersson M, Hilbertson A, Cenci MA (1999) Striatal fosB expression is causally linked with l-DOPA-induced abnormal involuntary movements and the associated upregulation of striatal prodynorphin mRNA in a rat model of Parkinson’s disease. Neurobiol Dis 6:461–474
Antonini A, Barone P, Bonuccelli U et al (2017) ICARUS study: prevalence and clinical features of impulse control disorders in Parkinson’s disease. J Neurol Neurosurg Psychiatry 88:317–324
Aubert I, Guigoni C, Håkansson K et al (2005) Increased D1 dopamine receptor signaling in levodopa-induced dyskinesia. Ann Neurol 57:17–26
Barger G, Dale HH (1910) Chemical structure and sympathomimetic action of amines. J Physiol 41:19–59
Barone P, Poewe W, Albrecht S et al (2010) Pramipexole for the treatment of depressive symptoms in patients with Parkinson’s disease: a randomised, double-blind, placebo-controlled trial. Lancet Neurol 9:573–580
Basma AN, Morris EJ, Nicklas WJ, Geller HM (1995) L-Dopa cytotoxicity to PC12 cells in culture is via its autoxidation. J Neurochem 64:825–832
Bastide MF, Dovero S, Charron G et al (2014) Immediate-early gene expression in structures outside the basal ganglia is associated to l-DOPA-induced dyskinesia. Neurobiol Dis 62:179–192
Bateup HS, Santini E, Shen W et al (2010) Distinct subclasses of medium spiny neurons differentially regulate striatal motor behaviors. Proc Natl Acad Sci U S A 107:14845–14850
Beaulieu J-M, Gainetdinov RR (2011) The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev 63:182–217
Berg D, Godau J, Trenkwalder C et al (2011) AFQ056 treatment of levodopa-induced dyskinesias: results of 2 randomized controlled trials. Mov Disord Off J Mov Disord Soc 26:1243–1250
Berke JD, Paletzki RF, Aronson GJ et al (1998) A complex program of striatal gene expression induced by dopaminergic stimulation. J Neurosci 18:5301–5310
Berthet A, Porras G, Doudnikoff E et al (2009) Pharmacological analysis demonstrates dramatic alteration of D1 dopamine receptor neuronal distribution in the rat analog of L-DOPA-induced dyskinesia. J Neurosci 29:4829–4835
Birkmayer W, Hornykiewicz O (1961) The L-3,4-dioxyphenylalanine (DOPA)-effect in Parkinson-akinesia. Wien Klin Wochenschr 73:787–788
Biundo R, Weis L, Abbruzzese G et al (2017) Impulse control disorders in advanced Parkinson’s disease with dyskinesia: the ALTHEA study. Mov Disord Off J Mov Disord Soc 32:1557–1565
Blanchet PJ, Calon F, Morissette M et al (2004) Relevance of the MPTP primate model in the study of dyskinesia priming mechanisms. Parkinsonism Relat Disord 10:297–304
Blunt S, Jenner P, Marsden CD (1991a) The effect of chronic L-dopa treatment on the recovery of motor function in 6-hydroxydopamine-lesioned rats receiving ventral mesencephalic grafts. Neuroscience 40:453–464
Blunt SB, Jenner P, Marsden CD (1991b) The effect of L-dopa and carbidopa treatment on the survival of rat fetal dopamine grafts assessed by tyrosine hydroxylase immunohistochemistry and [3H]mazindol autoradiography. Neuroscience 43:95–110
Bonifati V, Fabrizio E, Cipriani R et al (1994) Buspirone in levodopa-induced dyskinesias. Clin Neuropharmacol 17:73–82
Borgohain R, Szasz J, Stanzione P et al (2014a) Randomized trial of safinamide add-on to levodopa in Parkinson’s disease with motor fluctuations. Mov Disord Off J Mov Disord Soc 29:229–237
Borgohain R, Szasz J, Stanzione P et al (2014b) Two-year, randomized, controlled study of safinamide as add-on to levodopa in mid to late Parkinson’s disease. Mov Disord Off J Mov Disord Soc 29:1273–1280
Boudíková B, Szumlanski C, Maidak B, Weinshilboum R (1990) Human liver catechol-O-methyltransferase pharmacogenetics. Clin Pharmacol Ther 48:381–389
Braak H, Del Tredici K, Rüb U et al (2003) Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 24:197–211
Bravi D, Mouradian MM, Roberts JW et al (1994) Wearing-off fluctuations in Parkinson’s disease: contribution of postsynaptic mechanisms. Ann Neurol 36:27–31
Brewer JA, Potenza MN (2008) The neurobiology and genetics of impulse control disorders: relationships to drug addictions. Biochem Pharmacol 75:63
Brod LS, Aldred JL, Nutt JG (2012) Are high doses of carbidopa a concern? A randomized, clinical trial in Parkinson’s disease. Mov Disord Off J Mov Disord Soc 27:750–753
Calabresi P, Picconi B, Tozzi A, Di Filippo M (2007) Dopamine-mediated regulation of corticostriatal synaptic plasticity. Trends Neurosci 30:211–219
Calne DB, Teychenne PF, Leigh PN et al (1974) Treatment of parkinsonism with bromocriptine. Lancet Lond Engl 2:1355–1356
Calon F, Grondin R, Morissette M et al (2000) Molecular basis of levodopa-induced dyskinesias. Ann Neurol 47:S70–S78
Carlsson A, Lindqvist M, Magnusson T (1957) 3,4-Dihydroxyphenylalanine and 5-hydroxytryptophan as reserpine antagonists. Nature 180:1200
Carlsson A, Lindqvist M, Magnusson T, Waldeck B (1958) On the presence of 3-hydroxytyramine in brain. Science 127:471
Carta M, Carlsson T, Kirik D, Björklund A (2007) Dopamine released from 5-HT terminals is the cause of L-DOPA-induced dyskinesia in parkinsonian rats. Brain J Neurol 130:1819–1833
Cedarbaum JM (1989) The promise and limitations of controlled-release oral levodopa administration. Clin Neuropharmacol 12:147–166
Cenci MA (2007) Dopamine dysregulation of movement control in L-DOPA-induced dyskinesia. Trends Neurosci 30:236–243
Cenci MA (2014) Presynaptic mechanisms of L-DOPA-induced dyskinesia: the findings, the debate, and the therapeutic implications. Front Neurol 5:242
Cenci MA, Kalén P (2000) Serotonin release from mesencephalic raphe neurons grafted to the 5,7-dihydroxytryptamine-lesioned rat hippocampus: effects of behavioral activation and stress. Exp Neurol 164:351–361
Cenci MA, Konradi C (2010) Maladaptive striatal plasticity in L-DOPA-induced dyskinesia. Prog Brain Res 183:209–233
Cenci MA, Lundblad M (2006) Post- versus presynaptic plasticity in L-DOPA-induced dyskinesia. J Neurochem 99:381–392
Cenci MA, Tranberg A, Andersson M, Hilbertson A (1999) Changes in the regional and compartmental distribution of FosB- and JunB-like immunoreactivity induced in the dopamine-denervated rat striatum by acute or chronic L-dopa treatment. Neuroscience 94:515–527
Cepeda C, Levine MS (1998) Dopamine and N-methyl-D-aspartate receptor interactions in the neostriatum. Dev Neurosci 20:1–18
Cereda E, Barichella M, Pezzoli G (2010) Controlled-protein dietary regimens for Parkinson’s disease. Nutr Neurosci 13:29–32
Cerovic M, Bagetta V, Pendolino V et al (2015) Derangement of Ras-guanine nucleotide-releasing factor 1 (Ras-GRF1) and extracellular signal-regulated kinase (ERK) dependent striatal plasticity in L-DOPA-induced dyskinesia. Biol Psychiatry 77:106–115
Chalifoux JR, Carter AG (2010) GABAB receptors modulate NMDA receptor calcium signals in dendritic spines. Neuron 66:101–113
Charbonnier-Beaupel F, Malerbi M, Alcacer C et al (2015) Gene expression analyses identify Narp contribution in the development of L-DOPA-induced dyskinesia. J Neurosci 35:96–111
Chaudhuri KR, Schapira AH (2009) Non-motor symptoms of Parkinson’s disease: dopaminergic pathophysiology and treatment. Lancet Neurol 8:464–474
Cilia R, Benfante R, Asselta R et al (2016) Tryptophan hydroxylase type 2 variants modulate severity and outcome of addictive behaviors in Parkinson’s disease. Parkinsonism Relat Disord 29:96–103
Cohen G (1983) The pathobiology of Parkinson’s disease: biochemical aspects of dopamine neuron senescence. J Neural Transm Suppl 19:89–103
Comings DE, Gade-Andavolu R, Gonzalez N et al (2001) The additive effect of neurotransmitter genes in pathological gambling. Clin Genet 60:107–116
Contin M, Martinelli P (2010) Pharmacokinetics of levodopa. J Neurol 257:S253–S261
Contin M, Riva R, Martinelli P et al (1991) Effect of age on the pharmacokinetics of oral levodopa in patients with Parkinson’s disease. Eur J Clin Pharmacol 41:463–466
Contin M, Riva R, Martinelli P et al (1993) Pharmacodynamic modeling of oral levodopa: clinical application in Parkinson’s disease. Neurology 43:367–371
Contin M, Martinelli P, Mochi M et al (2005) Genetic polymorphism of catechol-O-methyltransferase and levodopa pharmacokinetic-pharmacodynamic pattern in patients with Parkinson’s disease. Mov Disord Off J Mov Disord Soc 20:734–739
Corvol J-C, Poewe W (2017) Pharmacogenetics of Parkinson’s disease in clinical practice. Mov Disord Clin Pract 4:173–180 4
Corvol J-C, Muriel M-P, Valjent E et al (2004) Persistent increase in olfactory type G-protein alpha subunit levels may underlie D1 receptor functional hypersensitivity in Parkinson disease. J Neurosci 24:7007–7014
Corvol J-C, Anzouan-Kacou J-B, Fauveau E et al (2007) Heart valve regurgitation, pergolide use, and parkinson disease: an observational study and meta-analysis. Arch Neurol 64:1721–1726
Corvol J-C, Bonnet C, Charbonnier-Beaupel F et al (2011) The COMT Val158Met polymorphism affects the response to entacapone in Parkinson’s disease: a randomized crossover clinical trial. Ann Neurol 69:111–118
Cotzias GC, Van Woert MH, Schiffer LM (1967) Aromatic amino acids and modification of parkinsonism. N Engl J Med 276:374–379
Cotzias GC, Papavasiliou PS, Gellene R (1969) Modification of parkinsonism—chronic treatment with L-dopa. N Engl J Med 280:337–345
Darmopil S, Martín AB, De Diego IR et al (2009) Genetic inactivation of dopamine D1 but not D2 receptors inhibits L-DOPA-induced dyskinesia and histone activation. Biol Psychiatry 66:603–613
Day M, Wang Z, Ding J et al (2006) Selective elimination of glutamatergic synapses on striatopallidal neurons in Parkinson disease models. Nat Neurosci 9:251–259
De Deurwaerdère P, Ramsay RR, Di Giovanni G (2017) Neurobiology and neuropharmacology of monoaminergic systems. Prog Neurobiol 151:1–3
Deak M, Clifton AD, Lucocq LM, Alessi DR (1998) Mitogen- and stress-activated protein kinase-1 (MSK1) is directly activated by MAPK and SAPK2/p38, and may mediate activation of CREB. EMBO J 17:4426–4441
Devos D, Lejeune S, Cormier-Dequaire F et al (2014) Dopa-decarboxylase gene polymorphisms affect the motor response to L-dopa in Parkinson’s disease. Parkinsonism Relat Disord 20:170–175
Diamond SG, Markham CH (1990) Longitudinal study of effects of early levodopa treatment on disability and mortality in Parkinson’s disease. Adv Neurol 53:399–403
Dumartin B, Doudnikoff E, Gonon F, Bloch B (2007) Differences in ultrastructural localization of dopaminergic D1 receptors between dorsal striatum and nucleus accumbens in the rat. Neurosci Lett 419:273–277
Dunah AW, Wang Y, Yasuda RP et al (2000) Alterations in subunit expression, composition, and phosphorylation of striatal N-methyl-D-aspartate glutamate receptors in a rat 6-hydroxydopamine model of Parkinson’s disease. Mol Pharmacol 57:342–352
Dunn KL, Espino PS, Drobic B et al (2005) The Ras-MAPK signal transduction pathway, cancer and chromatin remodeling. Biochem Cell Biol Biochim Biol Cell 83:1–14
Dupont E, Andersen A, Boas J et al (1996) Sustained-release Madopar HBS compared with standard Madopar in the long-term treatment of de novo parkinsonian patients. Acta Neurol Scand 93:14–20
Durif F, Debilly B, Galitzky M et al (2004) Clozapine improves dyskinesias in Parkinson disease: a double-blind, placebo-controlled study. Neurology 62:381–388
Ehringer H, Hornykiewicz O (1960) Distribution of noradrenaline and dopamine (3-hydroxytyramine) in the human brain and their behavior in diseases of the extrapyramidal system. Klin Wochenschr 38:1236–1239
Eisen SA, Lin N, Lyons MJ et al (1998) Familial influences on gambling behavior: an analysis of 3359 twin pairs. Addict Abingdon Engl 93:1375–1384
Elsworth JD, Roth RH (1997) Dopamine synthesis, uptake, metabolism, and receptors: relevance to gene therapy of Parkinson’s disease. Exp Neurol 144:4–9
Engeln M, Fasano S, Ahmed SH et al (2013) Levodopa gains psychostimulant-like properties after nigral dopaminergic loss. Ann Neurol 74:140–144
Fabbrini G, Brotchie JM, Grandas F et al (2007) Levodopa-induced dyskinesias. Mov Disord Off J Mov Disord Soc 22:1379–1389 quiz 1523
Fahn S (1996) Is levodopa toxic? Neurology 47:S184–S195
Fahn S (2015) The medical treatment of Parkinson disease from James Parkinson to George Cotzias. Mov Disord Off J Mov Disord Soc 30:4–18
Fahn S, Oakes D, Shoulson I et al (2004) Levodopa and the progression of Parkinson’s disease. N Engl J Med 351:2498–2508
Fantini ML, Macedo L, Zibetti M et al (2015) Increased risk of impulse control symptoms in Parkinson’s disease with REM sleep behaviour disorder. J Neurol Neurosurg Psychiatry 86:174–179
Fasano S, Bezard E, D’Antoni A et al (2010) Inhibition of Ras-guanine nucleotide-releasing factor 1 (Ras-GRF1) signaling in the striatum reverts motor symptoms associated with L-dopa-induced dyskinesia. Proc Natl Acad Sci U S A 107:21824–21829
Fasano A, Visanji NP, Liu LWC et al (2015) Gastrointestinal dysfunction in Parkinson’s disease. Lancet Neurol 14:625–639
Ferreira JJ, Katzenschlager R, Bloem BR et al (2013) Summary of the recommendations of the EFNS/MDS-ES review on therapeutic management of Parkinson’s disease. Eur J Neurol 20:5–15
Ferreira JJ, Lees A, Rocha J-F et al (2016) Opicapone as an adjunct to levodopa in patients with Parkinson’s disease and end-of-dose motor fluctuations: a randomised, double-blind, controlled trial. Lancet Neurol 15:154–165
Fieblinger T, Cenci MA (2015) Zooming in on the small: the plasticity of striatal dendritic spines in L-DOPA-induced dyskinesia. Mov Disord Off J Mov Disord Soc 30:484–493
Fieblinger T, Sebastianutto I, Alcacer C et al (2014) Mechanisms of dopamine D1 receptor-mediated ERK1/2 activation in the parkinsonian striatum and their modulation by metabotropic glutamate receptor type 5. J Neurosci 34:4728–4740
Fiorillo CD, Tobler PN, Schultz W (2003) Discrete coding of reward probability and uncertainty by dopamine neurons. Science 299:1898–1902
Fitzpatrick KM, Raschke J, Emborg ME (2009) Cell-based therapies for Parkinson’s disease: past, present, and future. Antioxid Redox Signal 11:2189–2208
Follett KA, Weaver FM, Stern M et al (2010) Pallidal versus subthalamic deep-brain stimulation for Parkinson’s disease. N Engl J Med 362:2077–2091
Fox SH, Chuang R, Brotchie JM (2009) Serotonin and Parkinson’s disease: on movement, mood, and madness. Mov Disord Off J Mov Disord Soc 24:1255–1266
Freed CR, Greene PE, Breeze RE et al (2001) Transplantation of embryonic dopamine neurons for severe Parkinson’s disease. N Engl J Med 344:710–719
Freitas ME, Fox SH (2016) Nondopaminergic treatments for Parkinson’s disease: current and future prospects. Neurodegener Dis Manag 6:249–268
Funk C (1911) On the chemical nature of the substance which cures polyneuritis in birds induced by a diet of polished rice. J Physiol 43:395–400
Gaspar P, Bloch B, Le Moine C (1995) D1 and D2 receptor gene expression in the rat frontal cortex: cellular localization in different classes of efferent neurons. Eur J Neurosci 7:1050–1063
Gerfen CR (2000) Molecular effects of dopamine on striatal-projection pathways. Trends Neurosci 23:S64–S70
Gerfen CR, Surmeier DJ (2011) Modulation of striatal projection systems by dopamine. Annu Rev Neurosci 34:441–466
Gerfen CR, Engber TM, Mahan LC et al (1990) D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science 250:1429–1432
Gerfen CR, Keefe KA, Gauda EB (1995) D1 and D2 dopamine receptor function in the striatum: coactivation of D1- and D2-dopamine receptors on separate populations of neurons results in potentiated immediate early gene response in D1-containing neurons. J Neurosci 15:8167–8176
Gerfen CR, Miyachi S, Paletzki R, Brown P (2002) D1 dopamine receptor supersensitivity in the dopamine-depleted striatum results from a switch in the regulation of ERK1/2/MAP kinase. J Neurosci 22:5042–5054
Gershanik OS (2015) Improving L-dopa therapy: the development of enzyme inhibitors. Mov Disord Off J Mov Disord Soc 30:103–113
Goetz CG, Olanow CW, Koller WC et al (1989) Multicenter study of autologous adrenal medullary transplantation to the corpus striatum in patients with advanced Parkinson’s disease. N Engl J Med 320:337–341
Gold SJ, Hoang CV, Potts BW et al (2007) RGS9-2 negatively modulates L-3,4-dihydroxyphenylalanine-induced dyskinesia in experimental Parkinson’s disease. J Neurosci 27:14338–14348
Greig SL, McKeage K (2016) Carbidopa/levodopa ER capsules (Rytary(®), NumientTM): a review in Parkinson’s disease. CNS Drugs 30:79–90
Guttman M, Léger G, Cedarbaum JM et al (1992) 3-O-methyldopa administration does not alter fluorodopa transport into the brain. Ann Neurol 31:638–643
Guzmán JN, Hernández A, Galarraga E et al (2003) Dopaminergic modulation of axon collaterals interconnecting spiny neurons of the rat striatum. J Neurosci 23:8931–8940
Håkansson K, Galdi S, Hendrick J et al (2006) Regulation of phosphorylation of the GluR1 AMPA receptor by dopamine D2 receptors. J Neurochem 96:482–488
Hallett PJ, Spoelgen R, Hyman BT et al (2006) Dopamine D1 activation potentiates striatal NMDA receptors by tyrosine phosphorylation-dependent subunit trafficking. J Neurosci 26:4690–4700
Han SK, Mytilineou C, Cohen G (1996) L-DOPA up-regulates glutathione and protects mesencephalic cultures against oxidative stress. J Neurochem 66:501–510
Harden DG, Grace AA (1995) Activation of dopamine cell firing by repeated L-DOPA administration to dopamine-depleted rats: its potential role in mediating the therapeutic response to L-DOPA treatment. J Neurosci 15:6157–6166
Hauser RA, Holford NHG (2002) Quantitative description of loss of clinical benefit following withdrawal of levodopa-carbidopa and bromocriptine in early Parkinson’s disease. Mov Disord Off J Mov Disord Soc 17:961–968
Hauser RA, Schapira AHV, Rascol O et al (2010) Randomized, double-blind, multicenter evaluation of pramipexole extended release once daily in early Parkinson’s disease. Mov Disord Off J Mov Disord Soc 25:2542–2549
Hauser RA, Cantillon M, Pourcher E et al (2011) Preladenant in patients with Parkinson’s disease and motor fluctuations: a phase 2, double-blind, randomised trial. Lancet Neurol 10:221–229
Hauser RA, Stocchi F, Rascol O et al (2015) Preladenant as an adjunctive therapy with levodopa in Parkinson disease: two randomized clinical trials and lessons learned. JAMA Neurol 72:1491–1500
Hefti F, Melamed E (1981) Dopamine release in rat striatum after administration of L-dope as studied with in vivo electrochemistry. Brain Res 225:333–346
Heiman M, Heilbut A, Francardo V et al (2014) Molecular adaptations of striatal spiny projection neurons during levodopa-induced dyskinesia. Proc Natl Acad Sci U S A 111:4578–4583
Hely MA, Morris JG, Traficante R et al (1999) The Sydney multicentre study of Parkinson’s disease: progression and mortality at 10 years. J Neurol Neurosurg Psychiatry 67:300–307
Higley MJ, Sabatini BL (2010) Competitive regulation of synaptic Ca2+ influx by D2 dopamine and A2A adenosine receptors. Nat Neurosci 13:958–966
Hoenicka J, García-Ruiz PJ, Ponce G et al (2015) The addiction-related gene ANKK1 in Parkinsonian patients with impulse control disorder. Neurotox Res 27:205–208
Hornykiewicz O (1958) The action of dopamine on the arterial blood pressure of the guinea-pig. Br J Pharmacol Chemother 13:91–94
Hornykiewicz O (1966) Dopamine (3-hydroxytyramine) and brain function. Pharmacol Rev 18:925–964
Huang CC, Hsu KS (2001) Progress in understanding the factors regulating reversibility of long-term potentiation. Rev Neurosci 12:51–68
Huot P, Johnston TH, Koprich JB et al (2013) The pharmacology of L-DOPA-induced dyskinesia in Parkinson’s disease. Pharmacol Rev 65:171–222
Hurley MJ, Mash DC, Jenner P (2001) Dopamine D(1) receptor expression in human basal ganglia and changes in Parkinson’s disease. Brain Res Mol Brain Res 87:271–279
Iwamoto K, Watanabe J, Yamada M et al (1987) Effect of age on gastrointestinal and hepatic first-pass effects of levodopa in rats. J Pharm Pharmacol 39:421–425
Jellinger KA (2012) Neuropathology of sporadic Parkinson’s disease: evaluation and changes of concepts. Mov Disord Off J Mov Disord Soc 27:8–30
Jonkers N, Sarre S, Ebinger G, Michotte Y (2001) Benserazide decreases central AADC activity, extracellular dopamine levels and levodopa decarboxylation in striatum of the rat. J Neural Transm Vienna Austria 1996 108:559–570
Kempster PA, Frankel JP, Bovingdon M et al (1989) Levodopa peripheral pharmacokinetics and duration of motor response in Parkinson’s disease. J Neurol Neurosurg Psychiatry 52:718–723
Kieburtz K, Olanow CW, Cohen Y, Oren S (2016) A randomized controlled clinical study to evaluate efficacy, safety and tolerability of SC L-dopa/carbidopa (ND0612H) infusion regimens in fluctuating PD patients [abstract]. Mov Disord 31(suppl 2). http://www.mdsabstracts.org/abstract/a-randomized-controlled-clinical-study-to-evaluate-efficacy-safety-and-tolerability-of-sc-l-dopacarbidopa-nd0612h-infusion-regimens-in-fluctuating-pd-patients/. Accessed 5 Feb 2018
Klebe S, Golmard J-L, Nalls MA et al (2013) The Val158Met COMT polymorphism is a modifier of the age at onset in Parkinson’s disease with a sexual dimorphism. J Neurol Neurosurg Psychiatry 84:666–673
Koller WC, Hutton JT, Tolosa E, Capilldeo R (1999) Immediate-release and controlled-release carbidopa/levodopa in PD: a 5-year randomized multicenter study. Carbidopa/Levodopa Study Group. Neurology 53:1012–1019
Kondo T, Mizuno Y, Japanese Istradefylline Study Group (2015) A long-term study of istradefylline safety and efficacy in patients with Parkinson disease. Clin Neuropharmacol 38:41–46
Kostic V, Przedborski S, Flaster E, Sternic N (1991) Early development of levodopa-induced dyskinesias and response fluctuations in young-onset Parkinson’s disease. Neurology 41:202–205
Kotecha SA, Oak JN, Jackson MF et al (2002) A D2 class dopamine receptor transactivates a receptor tyrosine kinase to inhibit NMDA receptor transmission. Neuron 35:1111–1122
Kraemmer J, Smith K, Weintraub D et al (2016) Clinical-genetic model predicts incident impulse control disorders in Parkinson’s disease. J Neurol Neurosurg Psychiatry 87:1106–1111
Krapivinsky G, Krapivinsky L, Manasian Y et al (2003) The NMDA receptor is coupled to the ERK pathway by a direct interaction between NR2B and RasGRF1. Neuron 40:775–784
Kravitz AV, Freeze BS, Parker PRL et al (2010) Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature 466:622–626
Kreitzer AC, Malenka RC (2007) Endocannabinoid-mediated rescue of striatal LTD and motor deficits in Parkinson’s disease models. Nature 445:643–647
Krishnamoorthy S, Rajan R, Banerjee M et al (2016) Dopamine D3 receptor Ser9Gly variant is associated with impulse control disorders in Parkinson’s disease patients. Parkinsonism Relat Disord 30:13–17
Kumakura Y, Cumming P (2009) PET studies of cerebral levodopa metabolism: a review of clinical findings and modeling approaches. Neurosci Rev J Bringing Neurobiol Neurol Psychiatry 15:635–650
Kumar R, Hauser RA, Mostillo J et al (2016) Mavoglurant (AFQ056) in combination with increased levodopa dosages in Parkinson’s disease patients. Int J Neurosci 126:20–24
Le Foll B, Goldberg SR, Sokoloff P (2005) The dopamine D3 receptor and drug dependence: effects on reward or beyond? Neuropharmacology 49:525–541
Lebel M, Chagniel L, Bureau G, Cyr M (2010) Striatal inhibition of PKA prevents levodopa-induced behavioural and molecular changes in the hemiparkinsonian rat. Neurobiol Dis 38:59–67
Lee FJS, Xue S, Pei L et al (2002a) Dual regulation of NMDA receptor functions by direct protein-protein interactions with the dopamine D1 receptor. Cell 111:219–230
Lee MS, Kim HS, Cho EK et al (2002b) COMT genotype and effectiveness of entacapone in patients with fluctuating Parkinson’s disease. Neurology 58:564–567
Lee WY, Yoon WT, Shin HY, Jeon SH, Rhee PL (2008) Helicobacter pylori infection and motor fluctuations in patients with Parkinson's disease. Mov Disord 23(12):1696–1700
Lee J-Y, Lee EK, Park SS et al (2009) Association of DRD3 and GRIN2B with impulse control and related behaviors in Parkinson’s disease. Mov Disord Off J Mov Disord Soc 24:1803–1810
Lee J-Y, Jeon BS, Kim H-J, Park S-S (2012) Genetic variant of HTR2A associates with risk of impulse control and repetitive behaviors in Parkinson’s disease. Parkinsonism Relat Disord 18:76–78
Leenders KL, Poewe WH, Palmer AJ et al (1986) Inhibition of L-[18F]fluorodopa uptake into human brain by amino acids demonstrated by positron emission tomography. Ann Neurol 20:258–262
Lees AJ, Stern GM (1981) Sustained bromocriptine therapy in previously untreated patients with Parkinson’s disease. J Neurol Neurosurg Psychiatry 44:1020–1023
Lesser RP, Fahn S, Snider SR et al (1979) Analysis of the clinical problems in parkinsonism and the complications of long-term levodopa therapy. Neurology 29:1253–1260
LeWitt PA (1993) Levodopa therapeutics: new treatment strategies. Neurology 43:S31–S37
LeWitt PA (2015) Levodopa therapy for Parkinson’s disease: pharmacokinetics and pharmacodynamics. Mov Disord Off J Mov Disord Soc 30:64–72
LeWitt PA, Nelson MV, Berchou RC et al (1989) Controlled-release carbidopa/levodopa (Sinemet 50/200 CR4): clinical and pharmacokinetic studies. Neurology 39:45–53 discussion 59
LeWitt PA, Hauser RA, Grosset DG et al (2016) A randomized trial of inhaled levodopa (CVT-301) for motor fluctuations in Parkinson’s disease. Mov Disord Off J Mov Disord Soc 31:1356–1365
Li Y-C, Xi D, Roman J et al (2009) Activation of glycogen synthase kinase-3 beta is required for hyperdopamine and D2 receptor-mediated inhibition of synaptic NMDA receptor function in the rat prefrontal cortex. J Neurosci 29:15551–15563
Limousin P, Pollak P, Benazzouz A et al (1995) Effect of parkinsonian signs and symptoms of bilateral subthalamic nucleus stimulation. Lancet Lond Engl 345:91–95
Lindvall O, Brundin P, Widner H et al (1990) Grafts of fetal dopamine neurons survive and improve motor function in Parkinson’s disease. Science 247:574–577
Lotti VJ, Porter CC (1970) Potentiation and inhbition of some central actions of L(-)-dopa by decarboxylase inhibitors. J Pharmacol Exp Ther 172:406–415
Lyras L, Zeng B-Y, McKenzie G et al (2002) Chronic high dose L-DOPA alone or in combination with the COMT inhibitor entacapone does not increase oxidative damage or impair the function of the nigro-striatal pathway in normal cynomologus monkeys. J Neural Transm Vienna Austria 1996 109:53–67
Madrazo I, Drucker-Colín R, Díaz V et al (1987) Open microsurgical autograft of adrenal medulla to the right caudate nucleus in two patients with intractable Parkinson’s disease. N Engl J Med 316:831–834
Maloney EM, Djamshidian A, O’Sullivan SS (2017) Phenomenology and epidemiology of impulsive-compulsive behaviours in Parkinson’s disease, atypical Parkinsonian disorders and non-Parkinsonian populations. J Neurol Sci 374:47–52
Männistö PT, Ulmanen I, Lundström K et al (1992) Characteristics of catechol O-methyl-transferase (COMT) and properties of selective COMT inhibitors. Prog Drug Res Fortschr Arzneimittelforschung Prog Rech Pharm 39:291–350
Marrinan S, Emmanuel AV, Burn DJ (2014) Delayed gastric emptying in Parkinson’s disease. Mov Disord Off J Mov Disord Soc 29:23–32
Marsden CD, Parkes JD (1977) Success and problems of long-term levodopa therapy in Parkinson’s disease. Lancet Lond Engl 1:345–349
Marshall JF, Navarrete R, Joyce JN (1989) Decreased striatal D1 binding density following mesotelencephalic 6-hydroxydopamine injections: an autoradiographic analysis. Brain Res 493:247–257
Martinez-Martin P, Schapira AHV, Stocchi F et al (2007) Prevalence of nonmotor symptoms in Parkinson’s disease in an international setting; study using nonmotor symptoms questionnaire in 545 patients. Mov Disord Off J Mov Disord Soc 22:1623–1629
Martinez-Martin P, Rodriguez-Blazquez C, Kurtis MM et al (2011) The impact of non-motor symptoms on health-related quality of life of patients with Parkinson’s disease. Mov Disord Off J Mov Disord Soc 26:399–406
Mazzella L, Yahr MD, Marinelli L et al (2005) Dyskinesias predict the onset of motor response fluctuations in patients with Parkinson’s disease on L-dopa monotherapy. Parkinsonism Relat Disord 11:151–155
Meiser J, Weindl D, Hiller K (2013) Complexity of dopamine metabolism. Cell Commun Signal 11:34
Mela F, Marti M, Dekundy A et al (2007) Antagonism of metabotropic glutamate receptor type 5 attenuates l-DOPA-induced dyskinesia and its molecular and neurochemical correlates in a rat model of Parkinson’s disease. J Neurochem 101:483–497
Mena I, Cotzias GC (1975) Protein intake and treatment of Parkinson’s disease with levodopa. N Engl J Med 292:181–184
Mena MA, Pardo B, Casarejos MJ et al (1992) Neurotoxicity of levodopa on catecholamine-rich neurons. Mov Disord Off J Mov Disord Soc 7:23–31
Menegoz M, Lau LF, Hervé D et al (1995) Tyrosine phosphorylation of NMDA receptor in rat striatum: effects of 6-OH-dopamine lesions. Neuroreport 7:125–128
Miyawaki E, Lyons K, Pahwa R et al (1997) Motor complications of chronic levodopa therapy in Parkinson’s disease. Clin Neuropharmacol 20:523–530
Mizuno Y, Kondo T, Japanese Istradefylline Study Group (2013) Adenosine A2A receptor antagonist istradefylline reduces daily OFF time in Parkinson’s disease. Mov Disord Off J Mov Disord Soc 28:1138–1141
Montagu KA (1957) Catechol compounds in rat tissues and in brains of different animals. Nature 180:244–245
Moreau C, Meguig S, Corvol J-C et al (2015) Polymorphism of the dopamine transporter type 1 gene modifies the treatment response in Parkinson’s disease. Brain J Neurol 138:1271–1283
Morissette M, Goulet M, Soghomonian JJ et al (1997) Preproenkephalin mRNA expression in the caudate-putamen of MPTP monkeys after chronic treatment with the D2 agonist U91356A in continuous or intermittent mode of administration: comparison with L-DOPA therapy. Brain Res Mol Brain Res 49:55–62
Mosharov EV, Borgkvist A, Sulzer D (2015) Presynaptic effects of levodopa and their possible role in dyskinesia. Mov Disord Off J Mov Disord Soc 30:45–53
Mouradian MM, Juncos JL, Fabbrini G, Chase TN (1987) Motor fluctuations in Parkinson’s disease: pathogenetic and therapeutic studies. Ann Neurol 22:475–479
Muenter MD, Tyce GM (1971) L-dopa therapy of Parkinson’s disease: plasma L-dopa concentration, therapeutic response, and side effects. Mayo Clin Proc 46:231–239
Murata M, Mizusawa H, Yamanouchi H, Kanazawa I (1996) Chronic levodopa therapy enhances dopa absorption: contribution to wearing-off. J Neural Transm Vienna Austria 1996 103:1177–1185
Murer MG, Dziewczapolski G, Menalled LB et al (1998) Chronic levodopa is not toxic for remaining dopamine neurons, but instead promotes their recovery, in rats with moderate nigrostriatal lesions. Ann Neurol 43:561–575
Mytilineou C, Han SK, Cohen G (1993) Toxic and protective effects of L-dopa on mesencephalic cell cultures. J Neurochem 61:1470–1478
Mytilineou C, Walker RH, JnoBaptiste R, Olanow CW (2003) Levodopa is toxic to dopamine neurons in an in vitro but not an in vivo model of oxidative stress. J Pharmacol Exp Ther 304:792–800
Nair VD, Olanow CW, Sealfon SC (2003) Activation of phosphoinositide 3-kinase by D2 receptor prevents apoptosis in dopaminergic cell lines. Biochem J 373:25–32
Ng KY, Chase TN, Colburn RW, Kopin IJ (1970) L-Dopa-induced release of cerebral monoamines. Science 170:76–77
Ng KY, Colburn RW, Kopin IJ (1971) Effects of L-dopa on efflux of cerebral monoamines from synaptosomes. Nature 230:331–332
Nutt JG (1987) On-off phenomenon: relation to levodopa pharmacokinetics and pharmacodynamics. Ann Neurol 22:535–540
Nutt JG, Holford NH (1996) The response to levodopa in Parkinson’s disease: imposing pharmacological law and order. Ann Neurol 39:561–573
Nutt JG, Woodward WR, Hammerstad JP et al (1984) The “on-off” phenomenon in Parkinson’s disease. Relation to levodopa absorption and transport. N Engl J Med 310:483–488
Nutt JG, Woodward WR, Anderson JL (1985) The effect of carbidopa on the pharmacokinetics of intravenously administered levodopa: the mechanism of action in the treatment of parkinsonism. Ann Neurol 18:537–543
Nutt JG, Carter JH, Lea ES, Woodward WR (1997) Motor fluctuations during continuous levodopa infusions in patients with Parkinson’s disease. Mov Disord Off J Mov Disord Soc 12:285–292
Nutt JG, Carter JH, Lea ES, Sexton GJ (2002) Evolution of the response to levodopa during the first 4 years of therapy. Ann Neurol 51:686–693
Nyholm D (2006) Pharmacokinetic optimisation in the treatment of Parkinson’s disease : an update. Clin Pharmacokinet 45:109–136
Nyholm D, Lennernäs H (2008) Irregular gastrointestinal drug absorption in Parkinson’s disease. Expert Opin Drug Metab Toxicol 4:193–203
Obeso JA, Vaamonde J, Grandas F et al (1989) Overcoming pharmacokinetic problems in the treatment of Parkinson’s disease. Mov Disord Off J Mov Disord Soc 4(Suppl 1):S70–S85
Oertel WH, Wolters E, Sampaio C et al (2006) Pergolide versus levodopa monotherapy in early Parkinson’s disease patients: the PELMOPET study. Mov Disord Off J Mov Disord Soc 21:343–353
Ogasahara S, Nishikawa Y, Takahashi M et al (1984) Dopamine metabolism in the central nervous system after discontinuation of L-dopa therapy in patients with Parkinson disease. J Neurol Sci 66:151–163
Oh JD, Russell DS, Vaughan CL et al (1998) Enhanced tyrosine phosphorylation of striatal NMDA receptor subunits: effect of dopaminergic denervation and L-DOPA administration. Brain Res 813:150–159
Olanow CW (2015) Levodopa: effect on cell death and the natural history of Parkinson’s disease. Mov Disord Off J Mov Disord Soc 30:37–44
Olanow CW, Goetz CG, Kordower JH et al (2003) A double-blind controlled trial of bilateral fetal nigral transplantation in Parkinson’s disease. Ann Neurol 54:403–414
Olanow CW, Obeso JA, Stocchi F (2006) Continuous dopamine-receptor treatment of Parkinson’s disease: scientific rationale and clinical implications. Lancet Neurol 5:677–687
Olanow CW, Rascol O, Hauser R et al (2009) A double-blind, delayed-start trial of rasagiline in Parkinson’s disease. N Engl J Med 361:1268–1278
Olanow CW, Kieburtz K, Odin P et al (2014) Continuous intrajejunal infusion of levodopa-carbidopa intestinal gel for patients with advanced Parkinson’s disease: a randomised, controlled, double-blind, double-dummy study. Lancet Neurol 13:141–149
Oreland L (1991) Monoamine oxidase, dopamine and Parkinson’s disease. Acta Neurol Scand Suppl 136:60–65
Ory-Magne F, Corvol J-C, Azulay J-P et al (2014) Withdrawing amantadine in dyskinetic patients with Parkinson disease: the AMANDYSK trial. Neurology 82:300–307
Pahwa R, Tanner CM, Hauser RA et al (2017) ADS-5102 (amantadine) extended-release capsules for levodopa-induced dyskinesia in Parkinson disease (EASE LID study): a randomized clinical trial. JAMA Neurol 74:941–949
Palfi S, Gurruchaga JM, Ralph GS et al (2014) Long-term safety and tolerability of ProSavin, a lentiviral vector-based gene therapy for Parkinson’s disease: a dose escalation, open-label, phase 1/2 trial. Lancet Lond Engl 383:1138–1146
Pålhagen S, Heinonen EH, Hägglund J et al (1998) Selegiline delays the onset of disability in de novo parkinsonian patients. Swedish Parkinson Study Group. Neurology 51:520–525
Papay K, Xie SX, Stern M et al (2014) Naltrexone for impulse control disorders in Parkinson disease: a placebo-controlled study. Neurology 83:826–833
Pardo B, Mena MA, Fahn S, García de Yébenes J (1993) Ascorbic acid protects against levodopa-induced neurotoxicity on a catecholamine-rich human neuroblastoma cell line. Mov Disord Off J Mov Disord Soc 8:278–284
Parkinson Study Group (1989) DATATOP: a multicenter controlled clinical trial in early Parkinson’s disease. Arch Neurol 46(10):1052–1060
Parkinson Study Group (2000) Pramipexole vs levodopa as initial treatment for Parkinson disease: a randomized controlled trial. Parkinson Study Group. JAMA 284:1931–1938
Parkinson Study Group (2002) Dopamine transporter brain imaging to assess the effects of pramipexole vs levodopa on Parkinson disease progression. JAMA 287:1653–1661
Parkkinen L, O’Sullivan SS, Kuoppamäki M et al (2011) Does levodopa accelerate the pathologic process in Parkinson disease brain? Neurology 77:1420–1426
Pascoli V, Besnard A, Hervé D et al (2011) Cyclic adenosine monophosphate-independent tyrosine phosphorylation of NR2B mediates cocaine-induced extracellular signal-regulated kinase activation. Biol Psychiatry 69:218–227
Pavón N, Martín AB, Mendialdua A, Moratalla R (2006) ERK phosphorylation and FosB expression are associated with L-DOPA-induced dyskinesia in hemiparkinsonian mice. Biol Psychiatry 59:64–74
Perry TL, Yong VW, Ito M et al (1984) Nigrostriatal dopaminergic neurons remain undamaged in rats given high doses of L-DOPA and carbidopa chronically. J Neurochem 43:990–993
Phelps ME (2000) Positron emission tomography provides molecular imaging of biological processes. Proc Natl Acad Sci U S A 97:9226–9233
Picconi B, Centonze D, Håkansson K et al (2003) Loss of bidirectional striatal synaptic plasticity in L-DOPA-induced dyskinesia. Nat Neurosci 6:501–506
Pifl C, Nanoff C, Schingnitz G et al (1992) Sensitization of dopamine-stimulated adenylyl cyclase in the striatum of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated rhesus monkeys and patients with idiopathic Parkinson’s disease. J Neurochem 58:1997–2004
Pilleri M, Antonini A (2015) Therapeutic strategies to prevent and manage dyskinesias in Parkinson’s disease. Expert Opin Drug Saf 14:281–294
Politis M, Niccolini F (2015) Serotonin in Parkinson’s disease. Behav Brain Res 277:136–145
Postuma RB, Lang AE, Munhoz RP et al (2012) Caffeine for treatment of Parkinson disease: a randomized controlled trial. Neurology 79:651–658
Przedborski S, Levivier M, Raftopoulos C et al (1995) Peripheral and central pharmacokinetics of apomorphine and its effect on dopamine metabolism in humans. Mov Disord Off J Mov Disord Soc 10:28–36
Quinn N, Parkes JD, Marsden CD (1984) Control of on/off phenomenon by continuous intravenous infusion of levodopa. Neurology 34:1131–1136
Rangel-Barajas C, Silva I, Lopéz-Santiago LM et al (2011) L-DOPA-induced dyskinesia in hemiparkinsonian rats is associated with up-regulation of adenylyl cyclase type V/VI and increased GABA release in the substantia nigra reticulata. Neurobiol Dis 41:51–61
Rascol O, Perez-Lloret S (2009) Rotigotine transdermal delivery for the treatment of Parkinson’s disease. Expert Opin Pharmacother 10:677–691
Rascol O, Brooks DJ, Korczyn AD et al (2000) A five-year study of the incidence of dyskinesia in patients with early Parkinson’s disease who were treated with ropinirole or levodopa. N Engl J Med 342:1484–1491
Rascol O, Brooks DJ, Melamed E et al (2005) Rasagiline as an adjunct to levodopa in patients with Parkinson’s disease and motor fluctuations (LARGO, lasting effect in adjunct therapy with rasagiline given once daily, study): a randomised, double-blind, parallel-group trial. Lancet Lond Engl 365:947–954
Rascol O, Perez-Lloret S, Ferreira JJ (2015) New treatments for levodopa-induced motor complications. Mov Disord Off J Mov Disord Soc 30:1451–1460
Reches A, Fahn S, Bhawan J (1982) Chronic dopa feeding of mice. Neurology 32:684–685
Rektorova I, Balaz M, Svatova J et al (2008) Effects of ropinirole on nonmotor symptoms of Parkinson disease: a prospective multicenter study. Clin Neuropharmacol 31:261–266
Riddle JL, Rokosik SL, Napier TC (2012) Pramipexole- and methamphetamine-induced reward-mediated behavior in a rodent model of Parkinson’s disease and controls. Behav Brain Res 233:15–23
Rinne UK, Bracco F, Chouza C et al (1998) Early treatment of Parkinson’s disease with cabergoline delays the onset of motor complications. Results of a double-blind levodopa controlled trial. The PKDS009 Study Group. Drugs 55 Suppl 1:23–30
Rivera A, Alberti I, Martín AB et al (2002) Molecular phenotype of rat striatal neurons expressing the dopamine D5 receptor subtype. Eur J Neurosci 16:2049–2058
Robertson DR, Wood ND, Everest H et al (1989) The effect of age on the pharmacokinetics of levodopa administered alone and in the presence of carbidopa. Br J Clin Pharmacol 28:61–69
Robertson DR, Renwick AG, Wood ND et al (1990a) The influence of levodopa on gastric emptying in man. Br J Clin Pharmacol 29:47–53
Robertson GS, Vincent SR, Fibiger HC (1990b) Striatonigral projection neurons contain D1 dopamine receptor-activated c-fos. Brain Res 523:288–290
Robertson DR, Renwick AG, Macklin B et al (1992) The influence of levodopa on gastric emptying in healthy elderly volunteers. Eur J Clin Pharmacol 42:409–412
Roche KW, O’Brien RJ, Mammen AL et al (1996) Characterization of multiple phosphorylation sites on the AMPA receptor GluR1 subunit. Neuron 16:1179–1188
Ruiz-DeDiego I, Naranjo JR, Hervé D, Moratalla R (2015) Dopaminergic regulation of olfactory type G-protein α subunit expression in the striatum. Mov Disord Off J Mov Disord Soc 30:1039–1049
Rylander D, Recchia A, Mela F et al (2009) Pharmacological modulation of glutamate transmission in a rat model of L-DOPA-induced dyskinesia: effects on motor behavior and striatal nuclear signaling. J Pharmacol Exp Ther 330:227–235
Santini E, Valjent E, Usiello A et al (2007) Critical involvement of cAMP/DARPP-32 and extracellular signal-regulated protein kinase signaling in L-DOPA-induced dyskinesia. J Neurosci 27:6995–7005
Santini E, Alcacer C, Cacciatore S et al (2009) L-DOPA activates ERK signaling and phosphorylates histone H3 in the striatonigral medium spiny neurons of hemiparkinsonian mice. J Neurochem 108:621–633
Santini E, Sgambato-Faure V, Li Q et al (2010) Distinct changes in cAMP and extracellular signal-regulated protein kinase signalling in L-DOPA-induced dyskinesia. PLoS One 5:e12322
Sasahara K, Nitanai T, Habara T et al (1981) Dosage form design for improvement of bioavailability of levodopa V: absorption and metabolism of levodopa in intestinal segments of dogs. J Pharm Sci 70:1157–1160
Schapira AHV (2002) Neuroprotection and dopamine agonists. Neurology 58:S9–18
Schrag A, Quinn N (2000) Dyskinesias and motor fluctuations in Parkinson’s disease. A community-based study. Brain J Neurol 123(Pt 11):2297–2305
Schuepbach WMM, Rau J, Knudsen K et al (2013) Neurostimulation for Parkinson’s disease with early motor complications. N Engl J Med 368:610–622
Schultz W (2007) Multiple dopamine functions at different time courses. Annu Rev Neurosci 30:259–288
Schwab RS, Amador LV, Lettvin JY (1951) Apomorphine in Parkinson’s disease. Trans Am Neurol Assoc 56:251–253
Seeman P (2015) Parkinson’s disease treatment may cause impulse-control disorder via dopamine D3 receptors. Synapse 69:183–189
Seppi K, Weintraub D, Coelho M et al (2011) The Movement Disorder Society evidence-based medicine review update: treatments for the non-motor symptoms of Parkinson’s disease. Mov Disord Off J Mov Disord Soc 26:S42–S80
Shepherd JD, Huganir RL (2007) The cell biology of synaptic plasticity: AMPA receptor trafficking. Annu Rev Cell Dev Biol 23:613–643
Shinotoh H, Hirayama K, Tateno Y (1993) Dopamine D1 and D2 receptors in Parkinson’s disease and striatonigral degeneration determined by PET. Adv Neurol 60:488–493
Siderowf A, Kurlan R (1999) Monoamine oxidase and catechol-O-methyltransferase inhibitors. Med Clin North Am 83:445–467
Skeberdis VA, Chevaleyre V, Lau CG et al (2006) Protein kinase A regulates calcium permeability of NMDA receptors. Nat Neurosci 9:501–510
Slutske WS, Zhu G, Meier MH, Martin NG (2010) Genetic and environmental influences on disordered gambling in men and women. Arch Gen Psychiatry 67:624–630
Smith AD, Bolam JP (1990) The neural network of the basal ganglia as revealed by the study of synaptic connections of identified neurones. Trends Neurosci 13:259–265
Snyder GL, Allen PB, Fienberg AA et al (2000) Regulation of phosphorylation of the GluR1 AMPA receptor in the neostriatum by dopamine and psychostimulants in vivo. J Neurosci 20:4480–4488
Sokoloff P, Le Foll B (2017) The dopamine D3 receptor, a quarter century later. Eur J Neurosci 45:2–19
Southorn PA, Powis G (1988) Free radicals in medicine. I. Chemical nature and biologic reactions. Mayo Clin Proc 63:381–389
Steiner H, Gerfen CR (1996) Dynorphin regulates D1 dopamine receptor-mediated responses in the striatum: relative contributions of pre- and postsynaptic mechanisms in dorsal and ventral striatum demonstrated by altered immediate-early gene induction. J Comp Neurol 376:530–541
Stocchi F, Hersh BP, Scott BL et al (2008) Ropinirole 24-hour prolonged release and ropinirole immediate release in early Parkinson’s disease: a randomized, double-blind, non-inferiority crossover study. Curr Med Res Opin 24:2883–2895
Stocchi F, Rascol O, Destee A et al (2013) AFQ056 in Parkinson patients with levodopa-induced dyskinesia: 13-week, randomized, dose-finding study. Mov Disord Off J Mov Disord Soc 28:1838–1846
Sulzer D, Pothos EN (2000) Regulation of quantal size by presynaptic mechanisms. Rev Neurosci 11:159–212
Svenningsson P, Rosenblad C, Af Edholm Arvidsson K et al (2015) Eltoprazine counteracts l-DOPA-induced dyskinesias in Parkinson’s disease: a dose-finding study. Brain J Neurol 138:963–973
Sweet RD, McDowell FH (1975) Five years’ treatment of Parkinson’s disease with levodopa. Therapeutic results and survival of 100 patients. Ann Intern Med 83:456–463
Tanner CM, Chhablani R, Goetz CG, Klawans HL (1985) Pergolide mesylate: lack of cardiac toxicity in patients with cardiac disease. Neurology 35:918–921
Taverna S, Canciani B, Pennartz CMA (2005) Dopamine D1-receptors modulate lateral inhibition between principal cells of the nucleus accumbens. J Neurophysiol 93:1816–1819
Tecuapetla F, Koós T, Tepper JM et al (2009) Differential dopaminergic modulation of neostriatal synaptic connections of striatopallidal axon collaterals. J Neurosci 29:8977–8990
Thiele SL, Chen B, Lo C et al (2014) Selective loss of bi-directional synaptic plasticity in the direct and indirect striatal output pathways accompanies generation of parkinsonism and l-DOPA induced dyskinesia in mouse models. Neurobiol Dis 71:334–344
Tison F, Keywood C, Wakefield M et al (2016) A phase 2A trial of the novel mGluR5-negative allosteric modulator dipraglurant for levodopa-induced dyskinesia in Parkinson’s disease. Mov Disord Off J Mov Disord Soc 31:1373–1380
Todorova A, Jenner P, Ray Chaudhuri K (2014) Non-motor Parkinson’s: integral to motor Parkinson’s, yet often neglected. Pract Neurol 14:310–322
Tong H, Gibb AJ (2008) Dopamine D1 receptor inhibition of NMDA receptor currents mediated by tyrosine kinase-dependent receptor trafficking in neonatal rat striatum. J Physiol 586:4693–4707
Tong J, Fitzmaurice PS, Ang LC et al (2004) Brain dopamine-stimulated adenylyl cyclase activity in Parkinson’s disease, multiple system atrophy, and progressive supranuclear palsy. Ann Neurol 55:125–129
Trenkwalder C, Chaudhuri KR, Martinez-Martin P et al (2015) Prolonged-release oxycodone–naloxone for treatment of severe pain in patients with Parkinson’s disease (PANDA): a double-blind, randomised, placebo-controlled trial. Lancet Neurol 14:1161–1170
Trenkwalder C, Berg D, Rascol O et al (2016) A placebo-controlled trial of AQW051 in patients with moderate to severe levodopa-induced dyskinesia. Mov Disord Off J Mov Disord Soc 31:1049–1054
Tritsch NX, Sabatini BL (2012) Dopaminergic modulation of synaptic transmission in cortex and striatum. Neuron 76:33–50
Turjanski N, Lees AJ, Brooks DJ (1997) In vivo studies on striatal dopamine D1 and D2 site binding in L-dopa-treated Parkinson’s disease patients with and without dyskinesias. Neurology 49:717–723
Valastro B, Dekundy A, Krogh M et al (2007) Proteomic analysis of striatal proteins in the rat model of L-DOPA-induced dyskinesia. J Neurochem 102:1395–1409
Vallelunga A, Flaibani R, Formento-Dojot P et al (2012) Role of genetic polymorphisms of the dopaminergic system in Parkinson’s disease patients with impulse control disorders. Parkinsonism Relat Disord 18:397–399
Van Camp G, Flamez A, Cosyns B et al (2004) Treatment of Parkinson’s disease with pergolide and relation to restrictive valvular heart disease. Lancet Lond Engl 363:1179–1183
Van Gerpen JA, Kumar N, Bower JH et al (2006) Levodopa-associated dyskinesia risk among Parkinson disease patients in Olmsted County, Minnesota, 1976–1990. Arch Neurol 63:205–209
Verhagen Metman L, Del Dotto P, van den Munckhof P et al (1998) Amantadine as treatment for dyskinesias and motor fluctuations in Parkinson’s disease. Neurology 50:1323–1326
Vijayakumar D, Jankovic J (2016) Drug-induced dyskinesia, part 1: treatment of levodopa-induced dyskinesia. Drugs 76:759–777
Voon V, Fox SH (2007) Medication-related impulse control and repetitive behaviors in Parkinson disease. Arch Neurol 64:1089–1096
Voon V, Pessiglione M, Brezing C et al (2010) Mechanisms underlying dopamine-mediated reward bias in compulsive behaviors. Neuron 65:135–142
Voon V, Mehta AR, Hallett M (2011) Impulse control disorders in Parkinson’s disease: recent advances. Curr Opin Neurol 24:324–330
Voon V, Napier TC, Frank MJ et al (2017) Impulse control disorders and levodopa-induced dyskinesias in Parkinson’s disease: an update. Lancet Neurol 16:238–250
Wade DN, Mearrick PT, Morris JL (1973) Active transport of L-dopa in the intestine. Nature 242:463–465
Waller DG, Usman F, Renwick AG et al (1991) Oral amino acids and gastric emptying: an investigation of the mechanism of levodopa-induced gastric stasis. Br J Clin Pharmacol 32:771–773
Wang Q, Zhang W (2016) Maladaptive synaptic plasticity in L-DOPA-induced dyskinesia. Front Neural Circ 10:105
Wang Q, Jolly JP, Surmeier JD et al (2001) Differential dependence of the D1 and D5 dopamine receptors on the G protein gamma 7 subunit for activation of adenylylcyclase. J Biol Chem 276:39386–39393
Wang W, Dever D, Lowe J et al (2012) Regulation of prefrontal excitatory neurotransmission by dopamine in the nucleus accumbens core. J Physiol 590:3743–3769
Watkins P (2000) COMT inhibitors and liver toxicity. Neurology 55:S51–S52 discussion S53-56
Weil-Malherbe H, Bone AD (1957) Intracellular distribution of catecholamines in the brain. Nature 180:1050–1051
Weintraub D, Koester J, Potenza MN et al (2010) Impulse control disorders in Parkinson disease: a cross-sectional study of 3090 patients. Arch Neurol 67:589–595
Weintraub D, Papay K, Siderowf A, Parkinson’s Progression Markers Initiative (2013) Screening for impulse control symptoms in patients with de novo Parkinson disease: a case-control study. Neurology 80:176–180
Westin JE, Vercammen L, Strome EM et al (2007) Spatiotemporal pattern of striatal ERK1/2 phosphorylation in a rat model of L-DOPA-induced dyskinesia and the role of dopamine D1 receptors. Biol Psychiatry 62:800–810
Whone AL, Watts RL, Stoessl AJ et al (2003) Slower progression of Parkinson’s disease with ropinirole versus levodopa: the REAL-PET study. Ann Neurol 54:93–101
Wickens JR, Arbuthnott GW (2005) Chapter IV Structural and functional interactions in the striatum at the receptor level. In: Dunnett SB, Bentivoglio M, Björklund A, Hökfelt T (eds) Handbook of chemical neuroanatomy, Dopamine, volume 21. Elsevier, pp 199–236. https://doi.org/10.1016/S0924-8196(05)80008-9
Witjas T, Eusebio A, Fluchère F, Azulay J-P (2012) Addictive behaviors and Parkinson’s disease. Rev Neurol (Paris) 168:624–633
Yahr MD, Duvoisin RC, Schear MJ et al (1969) Treatment of parkinsonism with levodopa. Arch Neurol 21:343–354
Yan Z, Surmeier DJ (1997) D5 dopamine receptors enhance Zn2+-sensitive GABA(A) currents in striatal cholinergic interneurons through a PKA/PP1 cascade. Neuron 19:1115–1126
Zainal Abidin S, Tan EL, Chan S-C et al (2015) DRD and GRIN2B polymorphisms and their association with the development of impulse control behaviour among Malaysian Parkinson’s disease patients. BMC Neurol 15:59
Zengin-Toktas Y, Authier N, Denizot H et al (2013) Motivational properties of D2 and D3 dopamine receptors agonists and cocaine, but not with D1 dopamine receptors agonist and L-dopa, in bilateral 6-OHDA-lesioned rat. Neuropharmacology 70:74–82
Zesiewicz TA, Sullivan KL, Arnulf I et al (2010) Practice parameter: treatment of nonmotor symptoms of Parkinson disease: report of the quality standards Subcommittee of the American Academy of Neurology. Neurology 74:924–931
Zhuang X, Belluscio L, Hen R (2000) G(olf)alpha mediates dopamine D1 receptor signaling. J Neurosci 20:RC91
Funding
The research leading to these results received funding from the programme ‘Investissements d’Avenir’ ANR-10-IAIHU-06 and from the Innovative Medicines Initiative Joint Undertaking under grant agreement no. 115,568, resources of which comprise financial contributions from the European Union’s Seventh Framework Programme (FP7/2007–2013) and EFPIA companies’ in kind contribution.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
J.C.C. is in receipt of research grants from the French Ministry of Health, Agence Nationale pour le Recherche, Michael J Fox Foundation and honoraria for scientific advice from BMS, Zambon, Pfizer, Ipsen, Abbvie, Amarantus, Clevexel unrelated to this work. L.L.M. has received research support grants from INSERM, JNLF, The L’Oreal Foundation; speech honoraria from CSL, Sanofi-Genzyme, Lundbeck Teva; and received travel funding from the Movement Disorders Society, ANAINF, Medtronic, Teva and AbbVie, outside the submitted work. H.Y., G.M., D.L.F.D.N. and F.C.B. have no conflict of interest.
Rights and permissions
About this article
Cite this article
You, H., Mariani, LL., Mangone, G. et al. Molecular basis of dopamine replacement therapy and its side effects in Parkinson’s disease. Cell Tissue Res 373, 111–135 (2018). https://doi.org/10.1007/s00441-018-2813-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-018-2813-2