Abstract
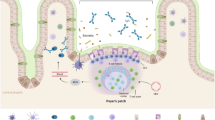
Microfold (M) cells in the follicle-associated epithelium (FAE) of Peyer’s patches contribute to the mucosal immune response by the transcytosis of microorganisms. The mechanism by which M cells take up microorganisms, and the functional proteins by which they do this, are not clear. In order to explore one such protein, we developed a 2H5-F3 monoclonal antibody (2H5-F3 mAb) through its binding to bovine M cells, and identified the antibody reactive molecule as cyclophilin A (Cyp-A). The localization patterns of Cyp-A were very similar to the localization pattern of cytokeratin (CK) 18-positive M cells. Cyp-A was identified at the luminal surface of CK18-positive M cells in bovine jejunal and ileal FAE. The membranous localization of Cyp-A in the bovine intestinal cell line (BIE cells) increased as cells differentiated toward M cells, as determined by flow cytometry analysis. Additionally, BIE cells released Cyp-A to the extracellular space and the differentiation of BIE cells to M cells increased the secretion of Cyp-A, as determined by western blotting. Accordingly, Cyp-A may be localized in M cells in the small intestinal epithelium of cattle. The rise of the membranous localization and secretion of Cyp-A by differentiation toward M cells indicates that Cyp-A has an important role in the function of M cells. While Cyp-A of the M cell membrane may contribute to the uptake of viruses with peptidyl-prolyl cis-trans isomerase activity, in the extracellular space Cyp-A may work as a chemokine and contribute to the distribution of immuno-competent cells.









Similar content being viewed by others
References
Andres PG, Beck PL, Mizoguchi E, Mizoguchi A, Bhan AK, Dawson T, Kuziel WA, Maeda N, MacDermott RP, Podolsky DK, Reinecker HC (2000) Mice with a selective deletion of the CC chemokine receptors 5 or 2 are protected from dextran sodium sulfate-mediated colitis: lack of CC chemokine receptor 5 expression results in a NK1.1 + lymphocyte-associated Th2-type immune response in the intestine. J Immunol 164:6303–12
Banks C, Bateman A, Payne R, Johnson P, Sheron N (2003) Chemokine expression in IBD. Mucosal Chemokine expression is unselectively increased in both ulcerative colitis and Crohn’s disease. J Pathol 199:28–35
Beyaz F, Asti RN (2004) Development of ileal Peyer’s patches and follicle associated epithelium in bovine fetuses. Anat Histol Embryol 33:172–179
Bjarnason I, MacPherson A, Hollander D (1995) Intestinal permeability: an overview. Gastroenterology 108:1566–1581
Bjerknes M, Cheng H (2005) Gastrointestinal stem cells. II. Intestinal stem cells. Am J Physiol Gastrointest Liver Physiol 289:G381–G387
Bockman DE (1983) Functional histology of appendix. Arch Histol Jpn 46:271–292
Brittan M, Wright NA (2004) Stem cell in gastrointestinal structure and neoplastic development. Gut 53:899–910
Bye WA, Allan CH, Trier JS (1984) Structure, distribution, and origin of M cells in Peyer’s patches of mouse ileum. Gastroenterology 86:789–801
Clark MA, Jepson MA, Simmons NL, Booth TA, Hirst BH (1993) Differential expression of lectin-binding sites defines mouse intestinal M-cells. J Histochem Cytochem 41:1679–1687
Clark MA, Hirst BH, Jepson MA (1998) M-cell surface beta1 integrin expression and invasion-mediated targeting of Yersinia pseudotuberculosis to mouse Peyer’s patch M cells. Infect Immun 66:1237–1243
Fischer G, Wittmann-Liebold B, Lang K, Kiefhaber T, Schmid FX (1989) Cyclophilin and Peptidyl-prolyl cis-trans isomerase are probably identical proteins. Nature 337:476–8
Flanagan WM, Corthésy B, Bram RJ, Crabtree GR (1991) Nuclear association of a T-cell transcription factor blocked by FK-506 and cyclosporin A. Nature 352:803–807
Fotopoulos G, Harari A, Michetti P, Trono D, Pantaleo G, Kraehenbuhl JP (2002) Transepithelial transport of HIV-1 by M cells is receptor-mediated. Proc Natl Acad Sci U S A 99:9410–9414
Fruman DA, Burakoff SJ, Bierer BE (1994) Immunophilins in protein folding and immunosuppression. FASEB J 8:391–400
Gebert A (1997) The role of M cells in the protection of mucosal membranes. Histochem Cell Biol 108:455–470
Gebert A, Rothkötter HJ, Pabst R (1994) Cytokeratin 18 is an M-cell marker in porcine Peyer’s patches. Cell Tissue Res 276:213–221
Giannasca PJ, Giannasca KT, Falk P, Gordon JI, Neutra MR (1994) Regional differences in glycoconjugates of intestinal M cells in mice: potential targets for mucosal vaccines. Am J Physiol Gastrointest Liver Physiol 267:G1108–G1121
Gullberg E, Söderholm JD (2006) Peyer’s patches and M cells as potential sites of the inflammatory onset in Crohn’s disease. Ann N Y Acad Sci 1072:218–32
Handschumacher RE, Harding MW, Rice J, Drugge RJ, Speicher DW (1984) Cyclophilin: a specific cytosolic binding protein for cyclosporin A. Science 226:544–547
Hase K, Murakami T, Takatsu H, Shimaoka T, Iimura M, Hamura K, Kawano K, Ohshima S, Chihara R, Itoh K, Yonehara S, Ohno H (2006) The membrane-bound chemokine CXCL16 expressed on follicle-associated epithelium and M cells mediates lympho-epithelial interaction in GALT. J Immunol 176:43–51
Hase K, Kawano K, Nochi T, Pontes GS, Fukuda S, Ebisawa M, Kadokura K, Tobe T, Fujimura Y, Kawano S, Yabashi A, Waguri S, Nakato G, Kimura S, Murakami T, Iimura M, Hamura K, Fukuoka S, Lowe AW, Itoh K, Kiyono H, Ohno H (2009) Uptake through glycoprotein 2 of FimH(+) bacteria by M cells initiates mucosal immune response. Nature 462:226–30
Heggebø R, Press CM, Gunnes G, Lie KI, Tranulis MA, Ulvund M, Groschup MH, Landsverk T (2000) Distribution of prion protein in the ileal Peyer’s patch of scrapie-free lambs and lambs naturally and experimentally exposed to the scrapie agent. J Gen Virol 81:2327–37
Heppner FL, Christ AD, Klein MA, Prinz M, Fried M, Kraehenbuhl JP, Aguzzi A (2001) Transepithelial prion transport by M cells. Nat Med 7:976–7
Hondo T, Kanaya T, Takakura I, Watanabe H, Takahashi Y, Nagasawa Y, Terada S, Ohwada S, Watanabe K, Kitazawa H, Rose MT, Yamaguchi T, Aso H (2011) Cytokeratin 18 is a specific marker of bovine intestinal M cell. Am J Physiol Gastrointest Liver Physiol 300:442–53
Jepson MA, Mason CM, Bennett MK, Simmons NL, Hirst BH (1992) Co-expression of vimentin and cytokeratins in M cells of rabbit intestinal lymphoid follicle-associated epithelium. Histochem J 24:33–39
Kanaya T, Miyazawa K, Takakura I, Itani W, Watanabe K, Ohwada S, Kitazawa H, Rose MT, McConochie HR, Okano H, Yamaguchi T, Aso H (2008) Differentiation of a murine intestinal epithelial cell line (MIE) toward the M cell lineage. Am J Physiol Gastrointest Liver Physiol 295:273–84
Kernéis S, Bogdanova A, Kraehenbuhl JP, Pringault E (1997) Conversion by Peyer’s patch lymphocytes of human enterocytes into M cells that transport bacteria. Science 277:949–52
Kraehenbuhl JP, Neutra MR (2000) Epithelial M cells: differentiation and function. Annu Rev Cell Dev Biol 16:301–332
Kucharzik T, Lügering A, Lügering N, Rautenberg K, Linnepe M, Cichon C, Reichelt R, Stoll R, Schmidt MA, Domschke W (2000) Characterization of M cell development during indomethacin-induced ileitis in rats. Aliment Pharmacol Ther 14:247–256
Landsverk T (1979) The gastrointestinal mucosa in young milk-fed calves. A scanning electron and light microscopic investigation. Acta Vet Scand 20:572–582
Landsverk T (1981a) Peyer’s patches and the follicle-associated epithelium in diarrheic calves. Pathomorphology, morphometry and acid phosphatase histochemistry. Acta Vet Scand 122:459–471
Landsverk T (1981b) The epithelium covering Peyer’s patches in young milk-fed celves. An ultrastructural and enzyme histochemical investigation. Acta Vet Scand 22:198–210
Landsverk T (1984) Is the ileo-caecal Peyer’s patch in ruminants a mammalian “bursa-equivalent”? Acta Pathol Microbiol Immunol Scand A 92:77–79
Landsverk T (1987) The follicle-associated epithelium of ileal Peyer’s patch in ruminants is distinguished by its shedding of 50 nm particles. Immunol Cell Biol 65:251–261
Landsverk T (1988) Phagocytosis and transcytosis by the follicle-associated epithelium of the ileal Peyer’s patch in calves. Immunol Cell Biol 66:261–268
MacDermott RP (1999) Chemokines in the inflammatory bowel diseases. J Clin Immunol 19:266–72
Madara JL, Nash S, Moore R, Atisook K (1990) Structure and function of the intestinal epithelial barrier in health and disease. Monogr Pathol 31:306–24
Marshman E, Booth C, Potten CS (2002) The intestinal epithelial stem cell. BioEssays 24:91–98
Miyazawa K, Aso H, Honda M, Kido T, Minashima T, Kanaya T, Watanabe K, Ohwada S, Rose MT, Yamaguchi T (2006) Identification of bovine dendritic cell phenotype from bovine peripheral blood. Res Vet Sci 81:40–45
Miyazawa K, Hondo T, Kanaya T, Tanaka S, Takakura I, Itani W, Rose MT, Kitazawa H, Yamaguchi T, Aso H (2010a) Characterization of newly established bovine intestinal epithelial cell line. Histochem Cell Biol 133:125–34
Miyazawa K, Kanaya T, Takakura I, Tanaka S, Hondo T, Watanabe H, Rose MT, Kitazawa H, Yamaguchi T, Katamine S, Nishida N, Aso H (2010b) Transcytosis of murine-adapted bovine spongiform encephalopathy agents in an in vitro bovine M cell model. J Virol 84:12285–91
Momotani E, Whipple DL, Thiermann AB, Cheville NF (1988) Role of M cells and macrophages in the entrance of Mycobacterium paratuberculosis into dome of ileal Peyer’s patches in calves. Vet Pathol 25:131–137
Neutra MR (1999) M cells in antigen sampling in mucosal tissues. Curr Top Microbiol Immunol 236:17–32
Neutra MR, Pringault E, Kraehenbuhl JP (1996) Antigen sampling across epithelial barriers and induction of mucosal immune responses. Annu Rev Immunol 14:275–300
Owen RL, Jones AL (1974) Epithelial cell specialization within human Peyer’s patches: an ultrastructural study of intestinal lymphoid follicles. Gastroenterology 66:189–203
Paar M, Liebler EM, Pohlenz JF (1992) Uptake of ferritin by follicle-associ- ated epithelium in the colon of calves. Vet Pathol 29:120–128
Parsons KR, Bland AP, Hall GA (1991) Follicle associated epithelium of the gut associated lymphoid tissue of cattle. Vet Pathol 28:22–29
Rautenberg K, Cichon C, Heyer G, Demel M, Schmidt MA (1996) Immunocytochemical characterization of the follicle-associated epithelium of Peyer’s patches: anti-cytokeratin 8 antibody (clone 4.1.18) as a molecular marker for rat M cells. Eur J Cell Biol 71:363–370
Regoli M, Bertelli E, Borghesi C, Nicoletti C (1995) Three-dimensional (3D-) reconstruction of M cells in rabbit Peyer’s patches; definition of the intraepithelial compartment of the follicle-associated epithelium. Anat Rec 243:19–26
Reynolds JD, Morris B (1983) The evolution and involution of Peyer’s patches in fetal and postnatal sheep. Eur J Immunol 13:627–635
Sharma R, Schumacher U, Adam E (1998) Lectin histochemistry reveals the appearance of M-cells in Peyer’s patches of SCID mice after syngeneic normal bone marrow transplantation. J Histochem Cytochem 46:143–148
Sherry B, Yarlett N, Strupp A, Cerami A (1992) Identification of cyclophilin as a proinflammatory secretory product of lipopolysaccharide-activated macrophages. Proc Natl Acad Sci U S A 89:3511–5
Song F, Zhang X, Ren XB, Zhu P, Xu J, Wang L, Li YF, Zhong N, Ru Q, Zhang DW, Jiang JL, Xia B, Chen ZN (2011) Cyclophilin A (CyPA) induces chemotaxis independent of its peptidylprolyl cis-trans isomerase activity: direct binding between CyPA and the ectodomain of CD147. J Biol Chem 286:8197–203
Suzuki J, Jin ZG, Meoli DF, Matoba T, Berk BC (2006) Cyclophilin A is secreted by a vesicular pathway in vascular smooth muscle cells. Circ Res 98:811–7
Takakura I, Miyazawa K, Kanaya T, Itani W, Watanabe K, Ohwada S, Watanabe H, Hondo T, Rose MT, Mori T, Sakaguchi S, Nishida N, Katamine S, Yamaguchi T, Aso H (2011) Orally administered prion protein is incorporated by m cells and spreads into lymphoid tissues with macrophages in prion protein knockout mice. Am J Pathol 179(3):1301–9
Torres-Medina A (1981) Morphologic characteristics of the epithelial surface of aggregated lymphoid follicles (Peyer’s patches) in the small intestine of newborn gnotobiotic calves and pigs. Am J Vet Res 42:232–236
Towers GJ, Hatziioannou T, Cowan S, Goff SP, Luban J, Bieniasz PD (2003) Cyclophilin A modulates the sensitivity of HIV-1 to host restriction factors. Nat Med 9:1138–43
Turner JR (2006) Molecular basis of epithelial barrier regulation: from basic mechanisms to clinical application. Am J Pathol 169:1901–9
Wang P, Heitman J (2005) The cyclophilins. Genome Biol 6:226
Zhao X, Sato A, Dela Cruz CS, Linehan M, Luegering A, Kucharzik T, Shirakawa AK, Marquez G, Farber JM, Williams I, Iwasaki A (2003) CCL9 is secreted by the follicle-associated epithelium and recruits dome region Peyer’s patch CD11b + dendritic cells. J Immunol 171:2797–803
Zhou D, Mei Q, Li J, He H (2012) Cyclophilin A and viral infections. Biochem Biophys Res Commun 424:647–50
Zhu P, Ding J, Zhou J, Dong WJ, Fan CM, Chen ZN (2005) Expression of CD147 on monocytes/macrophages in rheumatoid arthritis: its potential role in monocyte accumulation and matrix metalloproteinase production. Arthritis Res 7:1023–1033
Author contributions
T.H., S.S. and H.A. conception and design of research; T.H., S.S., Y.N., S.T., H.W., X.C., K.W., S.O. and H.A. performed experiments; T.H. and S.S. analyzed data; T.H., S.S., K.W., S.O., H.K., T.N. and H.A. interpreted results of experiments; T.H. and S.S. prepared figures; T.H., S.S., and S.T. drafted manuscript; T.H., S.S., M.T.R. and H.A. edited and revised the manuscript; H.A. approved final version of manuscript.
Grants
This study was supported by a Grant-in-Aid for Scientific Research (B) (no. 15H04586) and a Grant-in-Aid for Exploratory Research (no. 26660217) from the Japan Society for the Promotion of Science (JSPS).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
No conflicts of Interest, financial or otherwise, are declared by the author(s).
Additional information
Tetsuya Hondo, Shunsuke Someya and Yuya Nagasawa contributed equally to this work.
Rights and permissions
About this article
Cite this article
Hondo, T., Someya, S., Nagasawa, Y. et al. Cyclophilin A is a new M cell marker of bovine intestinal epithelium. Cell Tissue Res 364, 585–597 (2016). https://doi.org/10.1007/s00441-015-2342-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-015-2342-1