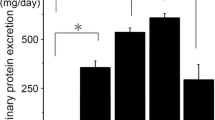
Abstract
Podoplanin was identified as a protein associated with the transformation of arborized foot processes of glomerular epithelial cells (podocytes) to flat feet. However, the function of podoplanin in the podocyte is not yet fully clarified. In this study, we analyzed the molecular nature of podoplanin, and its expression in rat nephrotic models and patients with minimal change nephrotic syndrome (MCNS). We demonstrated here that podoplanin has two forms: one contains abundant sialic acid and the other a lesser amount of sialic acid. Podoplanin bound ezrin to interact with the cytoskeleton. The silencing of podoplanin in cultured podocytes caused a change in the cell shape and the distribution of ezrin and actin. The expression of podoplanin was clearly reduced before the onset of proteinuria in puromycin aminonucleoside (PAN) nephropathy, a mimic of MCNS, and the decrease in the expression of podoplanin became more evident at the proteinuric stage. Podoplanin was detected in normal urine samples, and the amount of urinary podoplanin markedly increased on day 1 of PAN nephropathy. Urinary ezrin was also detected. The amount of the phosphorylated ezrin was reduced, while the amount of the podoplanin-interacting ezrin increased. The podoplanin expression was reduced in a patient with active-phase MCNS. It is conceivable that the alteration of the podoplanin–ezrin–cytoskeleton linkage is an important event of the podocyte injury in MCNS.






Similar content being viewed by others
References
Abbate M, Zoja C, Remuzzi G (2006) How does proteinuria cause progressive renal damage? J Am Soc Nephrol 17:2974–2984
Bertani T, Cutillo F, Zoja C, Broggini M, Remuzzi G (1986) Tubulo-interstitial lesions mediate renal damage in adriamycin glomerulopathy. Kidney Int 30:488–496
Bertram JF, Messina A, Ryan GB (1990) In vitro effects of puromycin aminonucleoside on the ultrastructure of rat glomerular podocytes. Cell Tissue Res 260:555–563
Breiteneder-Geleff S, Matsui K, Soleiman A, Meraner P, Poczewski H, Kalt R, Schaffner G, Kerjaschki D (1997) Podoplanin, novel 43-kd membrane protein of glomerular epithelial cells, is down-regulated in puromycin nephrosis. Am J Pathol 151:1141–1152
Breiteneder-Geleff S, Soleiman A, Kowalski H, Horvat R, Amann G, Kriehuber E, Diem K, Weninger W, Tschachler E, Alitalo K, Kerjaschki D (1999) Angiosarcomas express mixed endothelial phenotypes of blood and lymphatic capillaries: podoplanin as a specific marker for lymphatic endothelium. Am J Pathol 154:385–394
Charest PM, Roth J (1985) Localization of sialic acid in kidney glomeruli: regionalization in the podocyte plasma membrane and loss in experimental nephrosis. Proc Natl Acad Sci U S A 82:8508–8512
Clegg GR, Tyrrell C, McKechnie SR, Beers MF, Harrison D, McElroy MC (2005) Coexpression of RTI40 with alveolar epithelial type II cell proteins in lungs following injury: identification of alveolar intermediate cell types. Am J Physiol Lung Cell Mol Physiol 289:L382–390
Doublier S, Ruotsalainen V, Salvidio G, Lupia E, Biancone L, Conaldi PG, Reponen P, Tryggvason K, Camussi G (2001) Nephrin redistribution on podocytes is a potential mechanism for proteinuria in patients with primary acquired nephrotic syndrome. Am J Pathol 158:1723–1731
Fishman JA, Karnovsky MJ (1985) Effects of the aminonucleoside of puromycin on glomerular epithelial cells in vitro. Am J Pathol 118:398–407
Han GD, Koike H, Nakatsue T, Suzuki K, Yoneyama H, Narumi S, Kobayashi N, Mundel P, Shimizu F, Kawachi H (2003) IFN-inducible protein-10 has a differential role in podocyte during Thy 1.1 glomerulonephritis. J Am Soc Nephrol 14:3111–3126
Hashimoto T, Karasawa T, Saito A, Miyauchi N, Han GD, Hayasaka K, Shimizu F, Kawachi H (2007) Ephrin-B1 localizes at the slit diaphragm of the glomerular podocyte. Kidney Int 72:954–964
Hugo C, Nangaku M, Shankland SJ, Pichler R, Gordon K, Amieva MR, Couser WG, Furthmayr H, Johnson RJ (1998) The plasma membrane-actin linking protein, ezrin, is a glomerular epithelial cell marker in glomerulogenesis, in the adult kidney and in glomerular injury. Kidney Int 54:1934–1944
Kawachi H, Shimizu F (2000) Molecular composition and function of the slit diaphragm: nephrin, the molecule responsible for proteinuria. Clin Exp Nephrol 4:161–172
Kawachi H, Orikasa M, Matsui K, Iwanaga T, Toyabe S, Oite T, Shimizu F (1992) Epitope-specific induction of mesangial lesions with proteinuria by a MoAb against mesangial cell surface antigen. Clin Exp Immunol 88:399–404
Kawachi H, Koike H, Kurihara H, Yaoita E, Orikasa M, Shia MA, Sakai T, Yamamoto T, Salant DJ, Shimizu F (2000) Cloning of rat nephrin: expression in developing glomeruli and in proteinuric states. Kidney Int 57:1949–1961
Kawachi H, Koike H, Kurihara H, Sakai T, Shimizu F (2003) Cloning of rat homologue of podocin: expression in proteinuric states and in developing glomeruli. J Am Soc Nephrol 14:46–56
Kerjaschki D (2001) Caught flat-footed: podocyte damage and the molecular bases of focal glomerulosclerosis. J Clin Invest 108:1583–1587
Kerjaschki D, Sharkey DJ, Farquhar MG (1984) Identification and characterization of podocalyxin–the major sialoprotein of the renal glomerular epithelial cell. J Cell Biol 98:1591–1596
Kerjaschki D, Regele HM, Moosberger I, Nagy-Bojarski K, Watschinger B, Soleiman A, Birner P, Krieger S, Hovorka A, Silberhumer G, Laakkonen P, Petrova T, Langer B, Raab I (2004) Lymphatic neoangiogenesis in human kidney transplants is associated with immunologically active lymphocytic infiltrates. J Am Soc Nephrol 15:603–612
Kestila M, Lenkkeri U, Mannikko M, Lamerdin J, McCready P, Putaala H, Ruotsalainen V, Morita T, Nissinen M, Herva R, Kashtan CE, Peltonen L, Holmberg C, Olsen A, Tryggvason K (1998) Positionally cloned gene for a novel glomerular protein–nephrin–is mutated in congenital nephrotic syndrome. Mol Cell 1:575–582
Kriz W, Gretz N, Lemley KV (1998a) Progression of glomerular diseases: is the podocyte the culprit? Kidney Int 54:687–697
Kriz W, Kobayashi N, Elger M (1998b) New aspects of podocyte structure, function, and pathology. Clin Exp Nephrol 2:85–99
Macconi D, Bonomelli M, Benigni A, Plati T, Sangalli F, Longaretti L, Conti S, Kawachi H, Hill P, Remuzzi G, Remuzzi A (2006) Pathophysiologic implications of reduced podocyte number in a rat model of progressive glomerular injury. Am J Pathol 168:42–54
Mahtab EA, Wijffels MC, Van Den Akker NM, Hahurij ND, Lie-Venema H, Wisse LJ, Deruiter MC, Uhrin P, Zaujec J, Binder BR, Schalij MJ, Poelmann RE, Gittenberger-De Groot AC (2008) Cardiac malformations and myocardial abnormalities in podoplanin knockout mouse embryos: correlation with abnormal epicardial development. Dev Dyn 237:847–857
Martin-Villar E, Scholl FG, Gamallo C, Yurrita MM, Munoz-Guerra M, Cruces J, Quintanilla M (2005) Characterization of human PA2.26 antigen (T1alpha-2, podoplanin), a small membrane mucin induced in oral squamous cell carcinomas. Int J Cancer 113:899–910
Matsui K, Breiteneder-Geleff S, Kerjaschki D (1998a) Epitope-specific antibodies to the 43-kD glomerular membrane protein podoplanin cause proteinuria and rapid flattening of podocytes. J Am Soc Nephrol 9:2013–2026
Matsui T, Maeda M, Doi Y, Yonemura S, Amano M, Kaibuchi K, Tsukita S, Tsukita S (1998b) Rho-kinase phosphorylates COOH-terminal threonines of ezrin/radixin/moesin (ERM) proteins and regulates their head-to-tail association. J Cell Biol 140:647–657
Matsui K, Nagy-Bojarsky K, Laakkonen P, Krieger S, Mechtler K, Uchida S, Geleff S, Kang DH, Johnson RJ, Kerjaschki D (2003) Lymphatic microvessels in the rat remnant kidney model of renal fibrosis: aminopeptidase p and podoplanin are discriminatory markers for endothelial cells of blood and lymphatic vessels. J Am Soc Nephrol 14:1981–1989
Michael AF, Blau E, Vernier RL (1970) Glomerular polyanion. Alteration in aminonucleoside nephrosis. Lab Inves J Tech Methods Pathol 23:649–657
Miyauchi N, Saito A, Karasawa T, Harita Y, Suzuki K, Koike H, Han GD, Shimizu F, Kawachi H (2006) Synaptic vesicle protein 2B is expressed in podocyte, and its expression is altered in proteinuric glomeruli. J Am Soc Nephrol 17:2748–2759
Mundel P, Shankland SJ (2002) Podocyte biology and response to injury. J Am Soc Nephrol 13:3005–3015
Mundel P, Reiser J, Zuniga Mejia Borja A, Pavenstadt H, Davidson GR, Kriz W, Zeller R (1997) Rearrangements of the cytoskeleton and cell contacts induce process formation during differentiation of conditionally immortalized mouse podocyte cell lines. Exp Cell Res 236:248–258
Nakatsue T, Koike H, Han GD, Suzuki K, Miyauchi N, Yuan H, Salant DJ, Gejyo F, Shimizu F, Kawachi H (2005) Nephrin and podocin dissociate at the onset of proteinuria in experimental membranous nephropathy. Kidney Int 67:2239–2253
Okuda S, Oh Y, Tsuruda H, Onoyama K, Fujimi S, Fujishima M (1986) Adriamycin-induced nephropathy as a model of chronic progressive glomerular disease. Kidney Int 29:502–510
Orlando RA, Takeda T, Zak B, Schmieder S, Benoit VM, McQuistan T, Furthmayr H, Farquhar MG (2001) The glomerular epithelial cell anti-adhesin podocalyxin associates with the actin cytoskeleton through interactions with ezrin. J Am Soc Nephrol 12:1589–1598
Otaki Y, Miyauchi N, Higa M, Takada A, Kuroda T, Gejyo F, Shimizu F, Kawachi H (2008) Dissociation of NEPH1 from nephrin is involved in development of a rat model of focal segmental glomerulosclerosis. Am J Physiol Renal Physiol 295:F1376–1387
Pavenstadt H, Kriz W, Kretzler M (2003) Cell biology of the glomerular podocyte. Physiol Rev 83:253–307
Ryan GB, Karnovsky MJ (1975) An ultrastructural study of the mechanisms of proteinuria in aminonucleoside nephrosis. Kidney Int 8:219–232
Saito A, Miyauchi N, Hashimoto T, Karasawa T, Han GD, Kayaba M, Sumi T, Tomita M, Ikezumi Y, Suzuki K, Koitabashi Y, Shimizu F, Kawachi H (2011) Neurexin-1, a presynaptic adhesion molecule, localizes at the slit diaphragm of the glomerular podocytes in kidneys. Am J Physiol Regul Integr Comp Physiol 300:R340–348
Scholl FG, Gamallo C, Vilaro S, Quintanilla M (1999) Identification of PA2.26 antigen as a novel cell-surface mucin-type glycoprotein that induces plasma membrane extensions and increased motility in keratinocytes. J Cell Sci 112:4601–4613
Shankland SJ (2006) The podocyte’s response to injury: role in proteinuria and glomerulosclerosis. Kidney Int 69:2131–2147
Smoyer WE, Mundel P (1998) Regulation of podocyte structure during the development of nephrotic syndrome. J Mol Med 76:172–183
Somlo S, Mundel P (2000) Getting a foothold in nephrotic syndrome. Nat Genet 24:333–335
Takeda T, Go WY, Orlando RA, Farquhar MG (2000) Expression of podocalyxin inhibits cell-cell adhesion and modifies junctional properties in Madin-Darby canine kidney cells. Mol Biol Cell 11:3219–3232
Takeda T, McQuistan T, Orlando RA, Farquhar MG (2001) Loss of glomerular foot processes is associated with uncoupling of podocalyxin from the actin cytoskeleton. J Clin Invest 108:289–301
Tryggvason K (1999) Unraveling the mechanisms of glomerular ultrafiltration: nephrin, a key component of the slit diaphragm. J Am Soc Nephrol 10:2440–2445
Vanderbilt JN, Allen L, Gonzalez RF, Tigue Z, Edmondson J, Ansaldi D, Gillespie AM, Dobbs LG (2008) Directed expression of transgenes to alveolar type I cells in the mouse. Am J Respir Cell Mol Biol 39:253–262
Weening JJ, Rennke HG (1983) Glomerular permeability and polyanion in adriamycin nephrosis in the rat. Kidney Int 24:152–159
Wicki A, Lehembre F, Wick N, Hantusch B, Kerjaschki D, Christofori G (2006) Tumor invasion in the absence of epithelial-mesenchymal transition: podoplanin-mediated remodeling of the actin cytoskeleton. Cancer Cell 9:261–272
Zhu L, Zhou R, Mettler S, Wu T, Abbas A, Delaney J, Forte JG (2007) High turnover of ezrin T567 phosphorylation: conformation, activity, and cellular function. Am J Physiol Cell Physiol 293:C874–884
Acknowledgments
This work was supported by Grant-Aids for Scientific Research (B: 23390224 to H.K.) and Grant-Aid for Young Scientists (B: 24790839 to Y.F.) from the Ministry of Education, Culture, Sports, Science and Technology of Japan. The authors wish to thank Ms. Mutsumi Kayaba and Ms. Yukina Kitazawa for their excellent technical assistance and Mr. Masaaki Nameta for his help in the electron microscopic analysis.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Figure S1
a Full gels of western blot findings of podoplanin and actin of PAN nephropathy shown in Fig. 2d. b Full gels of western blot findings of podoplanin and actin of ADR nephropathy shown in Fig. 2d. c Full gels of western blot findings of podoplanin of PAN nephropathy (left panel) and ADR nephropathy (right panel) shown in Fig. 3. d The larger gels of western blot findings of podoplanin (right panel), actin (center) and ezrin (right panel) shown in Fig. 5d (GIF 108 kb)
Rights and permissions
About this article
Cite this article
Suzuki, K., Fukusumi, Y., Yamazaki, M. et al. Alteration in the podoplanin–ezrin–cytoskeleton linkage is an important initiation event of the podocyte injury in puromycin aminonucleoside nephropathy, a mimic of minimal change nephrotic syndrome. Cell Tissue Res 362, 201–213 (2015). https://doi.org/10.1007/s00441-015-2178-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-015-2178-8