Abstract
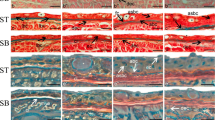
Flatfish metamorphosis is the most dramatic post-natal developmental event in teleosts. Thyroid hormones (TH), thyroxine (T4) and 3,3′-5′-triiodothyronine (T3) are the necessary and sufficient factors that induce and regulate flatfish metamorphosis. Most of the cellular and molecular action of TH is directed through the binding of T3 to thyroid nuclear receptors bound to promoters with consequent changes in the expression of target genes. The conversion of T4 to T3 and nuclear availability of T3 depends on the expression and activity of a family of 3 selenocysteine deiodinases that activate T4 into T3 or degrade T4 and T3. We have investigated the role of deiodinases in skin and muscle metamorphic changes in halibut. We show that, both at the whole body level and at the cellular level in muscle and skin of the Atlantic halibut (Hippoglossus hippoglossus) during metamorphosis, the coordination between activating (D2) and deactivating (D3) deiodinases expression is strongly correlated with the developmental TH-driven changes. The expression pattern of D2 and D3 in cells of both skin and muscle indicate that TH are necessary for the maintenance of larval metamorphic development and juvenile cell types in these tissues. No break in symmetry occurs in the expression of deiodinases and in metamorphic developmental changes occurring both in trunk skin and muscle. The findings that two of the major tissues in both larvae and juveniles maintain their symmetry throughout metamorphosis suggest that the asymmetric changes occurring during flatfish metamorphosis are restricted to the eye and head region.






Similar content being viewed by others
References
Altschul S, Gish W, Miller W, Myers E, Lipman D (1990) Basic local alignment search tool. J Mol Biol 215:403–410
Andersen Ø, Dahle SW, Nes S van, Bardal T, Tooming-Klunderud A, Kjørsvik E, Galloway TF (2009) Differential spatio-temporal expression and functional diversification of the myogenic regulatory factors MyoD1 and MyoD2 in Atlantic halibut (Hippoglossus hippoglossus).Comp Biochem Physiol B Biochem Mol Biol 154:93–101
Bao B, Yang G, Liu Z, Li S, Wang Z, Ren D (2005) Isolation of SFRS3 gene and its differential expression during metamorphosis involving eye migration of Japanese flounder Paralichthys olivaceus. Biochim Biophys Acta 1725:64–70
Bates JM, St Germain DL, Galton VA (1999) Expression profiles of the three iodothyronine deiodinases, D1, D2, and D3, in the developing rat. Endocrinology 140:844–851
Becker KB, Stephens KC, Davey JC, Schneider MJ, Galton VA (1997) The type 2 and type 3 iodothyronine deiodinases play important roles in coordinating development in Rana catesbeiana tadpoles. Endocrinology 138:2989–2997
Berry DL, Rose CS, Remo BF, Brown DD (1998) The expression pattern of thyroid hormone response genes in remodeling tadpole tissues defines distinct growth and resorption gene expression programs. Dev Biol 203:24–35
Bianco AC, Larsen PR (2005) Cellular and structural biology of the deiodinases. Thyroid 15:777–786
Bianco AC, Salvatore D, Gereben B, Berry MJ, Larsen PR (2002) Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocr Rev 23:38–89
Bres O, Plohman JC, Eales JG (2006) A cDNA for a putative type III deiodinase in the trout (Oncorhynchus mykiss): influence of holding conditions and thyroid hormone treatment on its hepatic expression. Gen Comp Endocrinol 145:92–100
Brown DD (2005) The role of deiodinases in amphibian metamorphosis. Thyroid 15:815–821
Brown DD, Cai L (2007) Amphibian metamorphosis. Dev Dyn 306:20–33
Buettner C, Harney JW, Larsen PR (1998) The 3′-untranslated region of human type 2 iodothyronine deiodinase mRNA contains a functional selenocysteine insertion sequence element. J Biol Chem 273:33374–33378
Caceres JF, Kornblihtt AR (2002) Alternative splicing: multiple control mechanisms and involvement in human disease. Trends Genet 18:186–193
Cai L, Brown DD (2004) Expression of type II iodothyronine deiodinase marks the time that a tissue responds to thyroid hormone-induced metamorphosis in Xenopus laevis. Dev Biol 266:87–95
Campinho MA, Sweeney GE, Power DM (2006) Regulation of troponin T expression by thyroid hormones during muscle development in sea bream (Sparus auratus, Linnaeus). J Exp Biol 209:4751–4767
Campinho MA, Silva N, Nowell M, Llewellyn L, Sweeney G, Power DM (2007a) Troponin T isoform expression is modulated during Atlantic halibut metamorphosis. BMC Dev Biol 7:71
Campinho MA, Silva N, Sweeney GE, Power DM (2007b) Molecular, cellular and histological changes in skin from a larval to an adult phenotype during bony fish metamorphosis. Cell Tissue Res 327:267–284
Campinho MA, Galay-Burgos M, Sweeney GE, Power DM (2010) Coordination of deiodinase and thyroid hormone receptor expression during the larval to juvenile transition in sea bream (Sparus aurata, Linnaeus). Gen Comp Endocrinol 165:181–194
Campos-Barros A, Amma LL, Faris JS, Shailam R, Kelley MW, Forrest D (2000) Type 2 iodothyronine deiodinase expression in the cochlea before the onset of hearing. Proc Natl Acad Sci USA 97:1287–1292
Croteau W, Whittemore SL, Schneider MJ, St Germain DL (1995) Cloning and expression of a cDNA for a mammalian type III iodothyronine deiodinase. J Biol Chem 270:16569–16575
Croteau W, Davey JC, Galton VA, St Germain DL (1996) Cloning of the mammalian type II iodothyronine deiodinase. A selenoprotein differentially expressed and regulated in human and rat brain and other tissues. J Clin Invest 98:405–417
Davey JC, Schneider MJ, Becker KB, Galton VA (1999) Cloning of a 5.8 kb cDNA for a mouse type 2 deiodinase. Endocrinology 140:1022–1025
Dentice M, Marsili A, Ambrosio R, Guardiola O, Sibilio A, Paik J-H, Minchiotti G, DePinho RA, Fenzi G, Larsen PR, Salvatore D (2010) The FoxO3/type 2 deiodinase pathway is required for normal mouse myogenesis and muscle regeneration. J Clin Invest 120:4021–4030
Einarsdóttir IE, Silva N, Power DM, Smáradóttirc H, Bjornssona BT (2006) Thyroid and pituitary gland development from hatching through metamorphosis of a teleost flatfish, the Atlantic halibut. Anat Embryol 211:47–60
Fagegaltier D, Lescure A, Walczak R, Carbon P, Krol A (2000) Structural analysis of new local features in SECIS RNA hairpins. Nucl Acids Res 28:2679–2689
Fitch WM (1971) Rate of change of concomitantly variable codons. J Mol Evol 1:84–96
Forrest D, Reh TA, Rusch A (2002) Neurodevelopmental control by thyroid hormone receptors. Curr Opin Neurobiol 12:49–56
Galay-Burgos M, Power DM, Llewellyn L, Sweeney GE (2008) Thyroid hormone receptor expression during metamorphosis of Atlantic halibut (Hippoglossus hippoglossus). Mol Cell Endocrinol 281:56–63
Galton VA (2005) The roles of the iodothyronine deiodinases in mammalian development. Thyroid 15:823–834
Gereben B, Zavacki AM, Ribich S, Kim BW, Huang SA, Simonides WS, Zeold A, Bianco AC (2008) Cellular and molecular basis of deiodinase-regulated thyroid hormone signaling. Endocr Rev 29:898–938
Harvey CB, Williams GR (2002) Mechanism of thyroid hormone action. Thyroid 12:441–446
Hernandez A, Lyon GJ, Schneider MJ, St Germain DL (1999) Isolation and characterization of the mouse gene for the type 3 iodothyronine deiodinase. Endocrinology 140:124–130
Huang TS, Chopra IJ, Boado R, Soloman DH, Chua Teco GN (1988) Thyroxine inner ring monodeiodinating activity in fetal tissues of the rat. Pediatr Res 23:196–199
Huang H, Marsh-Armstrong N, Brown DD (1999) Metamorphosis is inhibited in transgenic Xenopus laevis tadpoles that overexpress type III deiodinase. Proc Natl Acad Sci USA 96:962–967
Huang H, Cai L, Remo BF, Brown DD (2001) Timing of metamorphosis and the onset of the negative feedback loop between the thyroid gland and the pituitary is controlled by type II iodothyronine deiodinase in Xenopus laevis. Proc Natl Acad Sci USA 98:7348–7353
Huang MP, Rodgers KA, O’Mara R, Mehta M, Abuzahra HS, Tannenbaum AD, Persons K, Holick MF, Safer JD (2011) The thyroid hormone degrading type 3 deiodinase is the primary deiodinase active in murine epidermis. Thyroid 21:1263–1268
Infante C, Manchado M, Asensio E, Canavate J (2007) Molecular characterization, gene expression and dependence on thyroid hormones of two type I keratin genes (sseKer1 and sseKer2) in the flatfish Senegalese sole (Solea senegalensis Kaup). BMC Dev Biol 7:118
Ishida Y, Suzuki K, Utoh R, Obara M, Yoshizato K (2003) Molecular identification of the skin transformation center of anuran larval skin using genes of Rana adult keratin (RAK) and SPARC as probes. Dev Growth Differ 45:515–526
Isorna E, Obregon M-J, Calvo RM, Vázquez R, Pendón C, Falcón J, Muñoz-Cueto JA (2009) Iodothyronine deiodinases and thyroid hormone receptors regulation during flatfish (Solea senegalensis) metamorphosis. J Exp Zool B Mol Dev Evol 312B:231–246
Itoh K, Watanabe K, Wu X, Suzuki T (2010) Three members of the iodothyronine deiodinase family, dio1, dio2 and dio3, are expressed in spatially and temporally specific patterns during metamorphosis of the flounder, Paralichthys olivaceus. Zool Sci 27:574–580
Johnston IA (1994) Development and plasticity of fish muscle with growth. Basic Appl Myol 4:353–368
Johnston IA, Cole NJ, Vieira VLA, Davidson I (1997) Temperature and developmental plasticity of muscle phenotype in herring larvae. J Exp Biol 200:849–868
Johnston IA, Cole NJ, Abercromby M, Vieira VLA (1998) Embryonic temperature modulates muscle growth characteristics in larval and juvenile herring. J Exp Biol 201:623–646
Kawahara A, Gohda Y, Hikosaka A (1999) Role of type III iodothyronine 5-deiodinase gene expression in temporal regulation of Xenopus metamorphosis. Dev Growth Differ 41:365–373
Klaren PH, Haasdijk R, Metz JR, Nitsch LM, Darras VM, Van der Geyten S, Flik G (2005) Characterization of an iodothyronine 5′-deiodinase in gilthead seabream (Sparus auratus) that is inhibited by dithiothreitol. Endocrinology 146:5621–5630
Klaren PHM, Wunderink YS, Yfera M, Mancera JM, Flik G (2008) The thyroid gland and thyroid hormones in Senegalese sole (Solea senegalensis) during early development and metamorphosis. Gen Comp Endocrinol 155:686–694
Köhrle J (2000) The selenoenzyme family of deiodinase isozymes controls local thyroid hormone availability. Rev Endocr Metab Disord 1:49–58
Kollmus H, Flohe L, McCarthy JE (1996) Analysis of eukaryotic mRNA structures directing cotranslational incorporation of selenocysteine. Nucleic Acids Res 24:1195–1201
Kryukov GV, Castellano S, Novoselov SV, Lobanov AV, Zehtab O, Guigo R, Gladyshev VN (2003) Characterization of mammalian selenoproteomes. Science 300:1439–1443
Kuiper GGJM, Kester MHA, Peeters RP, Visser TJ (2005) Biochemical mechanisms of thyroid hormone deiodination. Thyroid 15:787–798
Lambert A, Lescure A, Gautheret D (2002) A survey of metazoan selenocysteine insertion sequences. Biochimie 84:953–959
Leonard DM, Stachelek SJ, Safran M, Farwell AP, Kowalik TF, Leonard JL (2000) Cloning, expression, and functional characterization of the substrate binding subunit of rat type II iodothyronine 5′-deiodinase. J Biol Chem 275:25194–25201
Llewellyn L, Ramsurn VP, Sweeney GE, Wigham T, Power DM (1998) Expression of thyroid hormone receptor during early development of the sea bream (Sparus aurata). Ann N Y Acad Sci 839:610–611
Mascarello F, Rowlerson A, Radaelli G, Scapolo PA, Veggetti A (1995) Differentiation and growth of muscle in the fish Sparus aurata (L). I. Myosin expression and organization of fibre types in lateral muscle from hatching to adult. J Muscle Res Cell Motil 16:213–222
Mathisen PM, Miller L (1987) Thyroid hormone induction of keratin genes: a two-step activation of gene expression during development. Genes Dev 1:1107–1117
Mathisen PM, Miller L (1989) Thyroid hormone induces constitutive keratin gene expression during Xenopus laevis development. Mol Cell Biol 9:1823–1831
Matlin AJ, Clark F, Smith CW (2005) Understanding alternative splicing: towards a cellular code. Nat Rev Mol Cell Biol 6:386–398
Mol KA, Van der Geyten S, Burel C, Kuhn ER, Boujard T, Darras VM (1998) Comparative study of iodothyronine outer ring and inner ring deiodination activities in five teleostean fishes. Fish Physiol Biochem 18:253–266
Moutou KA, Canario AV, Mamuris Z, Power DM (2001) Molecular cloning and sequence of Sparus aurata skeletal myosin light chains expressed in white muscle: developmental expression and thyroid regulation. J Exp Biol 204:3009–3018
Ng L, Goodyear RJ, Woods CA, Schneider MJ, Diamond E, Richardson GP, Kelley MW, Germain DLS, Galton VA, Forrest D (2004) Hearing loss and retarded cochlear development in mice lacking type 2 iodothyronine deiodinase. Proc Natl Acad Sci USA 101:3474–3479
Orozco A, Jeziorski MC, Linser PJ, Greenberg RM, Valverde RC (2002) Cloning of the gene and complete cDNA encoding a type 2 deiodinase from Fundulus heteroclitus. Gen Comp Endocrinol 128:162–167
Orozco A, Villalobos P, Jeziorski MC, Valverde RC (2003) The liver of Fundulus heteroclitus expresses deiodinase type 1 mRNA. Gen Comp Endocrinol 130:84–91
Radoja N, Diaz DV, Minars TJ, Freedberg IM, Blumenberg M, Tomic-Canic M (1997) Specific organization of the negative response elements for retinoic acid and thyroid hormone receptors in keratin gene family. J Invest Dermatol 109:566–572
Radoja N, Stojadinovic O, Waseem A, Tomic-Canic M, Milisavljevic V, Teebor S, Blumenberg M (2004) Thyroid hormones and gamma interferon specifically increase K15 keratin gene transcription. Mol Cell Biol 24:3168–3179
Richard K, Hume R, Kaptein E, Sanders JP, Toor H van, De Herder WW, Hollander JC den, Krenning EP, Visser TJ (1998) Ontogeny of iodothyronine deiodinases in human liver. J Clin Endocrinol Metab 83:2868–2874
Rowlerson A, Mascarello F, Radaelli G, Veggetti A (1995) Differentiation and growth of muscle in the fish Sparus aurata (L). II. Hyperplastic and hypertrophic growth of lateral muscle from hatching to adult. J Muscle Res Cell Motil 16:223–236
Sæle Ø, Solbakken J, Watanabe K, Hamre K, Power DM, Pittman K (2004) Staging of Atlantic halibut (Hippoglossus hippoglossus L.) from first feeding through metamorphosis, including cranial ossification independent of eye migration. Aquaculture 239:445–465
Salvatore D, Low SC, Berry M, Maia AL, Harney JW, Croteau W, St Germain DL, Larsen PR (1995) Type 3 iodothyronine deiodinase: cloning, in vitro expression, and functional analysis of the placental selenoenzyme. J Clin Invest 96:2421–2430
Sanders JP, Van der Geyten S, Kaptein E, Darras VM, Kuhn ER, Leonard JL, Visser TJ (1997) Characterization of a propylthiouracil-insensitive type I iodothyronine deiodinase. Endocrinology 138:5153–5160
Sanders JP, Van der Geyten S, Kaptein E, Darras VM, Kuhn ER, Leonard JL, Visser TJ (1999) Cloning and characterization of type III iodothyronine deiodinase from the fish Oreochromis niloticus. Endocrinology 140:3666–3673
Shin C, Manley JL (2004) Cell signalling and the control of pre-mRNA splicing. Nat Rev Mol Cell Biol 5:727–738
Shintani N, Nohira T, Hikosaka A, Kawahara A (2002) Tissue-specific regulation of type III iodothyronine 5-deiodinase gene expression mediates the effects of prolactin and growth hormone in Xenopus metamorphosis. Dev Growth Differ 44:327–335
Sinha S, Degenstein L, Copenhaver C, Fuchs E (2000) Defining the regulatory factors required for epidermal gene expression. Mol Cell Biol 20:2543–2555
St Germain DL, Schwartzman RA, Croteau W, Kanamori A, Wang Z, Brown DD, Galton VA (1994) A thyroid hormone-regulated gene in Xenopus laevis encodes a type III iodothyronine 5-deiodinase. Proc Natl Acad Sci USA 91:7767–7771
Sutija M, Longhurst TJ, Joss JMP (2003) Deiodinase type II and tissue specific mRNA alternative splicing in the Australian lungfish, Neoceratodus forsteri. Gen Comp Endocrinol 132:409–417
Suzuki K, Sato K, Katsu K, Hayashita H, Bach Kristensen D, Yoshizato K (2001) Novel Rana keratin genes and their expression during larval to adult epidermal conversion in bullfrog tadpoles. Differentiation 68:44–54
Suzuki K-i, Utoh R, Kotani K, Obara M, Yoshizato K (2002) Lineage of anuran epidermal basal cells and their differentiation potential in relation to metamorphic skin remodeling. Dev Growth Differ 44:225–238
Swofford DL, Waddell PJ, Huelsenbeck JP, Foster PG, Lewis PO, Rogers JS (2001) Bias in phylogenetic estimation and its relevance to the choice between parsimony and likelihood methods. Syst Biol 50:525–539
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739
Thompson J, Gibson T, Plewniak F, Jeanmougin F, Higgins D (1997) The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25:4876–4882
Tomic-Canic M, Sunjevaric I, Freedberg IM, Blumenberg M (1992) Identification of the retinoic acid and thyroid hormone receptor-responsive element in the human K14 keratin gene. J Invest Dermatol 99:842–847
Tomic-Canic M, Day D, Samuels HH, Freedberg IM, Blumenberg M (1996a) Novel regulation of keratin gene expression by thyroid hormone and retinoid receptors. J Biol Chem 271:1416–1423
Tomic-Canic M, Freedberg IM, Blumenberg M (1996b) Codominant regulation of keratin gene expression by cell surface receptors and nuclear receptors. Exp Cell Res 224:96–102
Valverde C, Croteau W, Lafleur GJ, Orozco A, Germain DL (1997) Cloning and expression of a 5′-iodothyronine deiodinase from the liver of Fundulus heteroclitus. Endocrinology 138:642–648
Veggetti A, Mascarello F, Scapolo PA, Rowlerson A, Candia-Carnevali MD (1993) Muscle growth and myosin isoform transitions during development of a small teleost fish, Poecilia reticulata (Peters) (Atheriniformes, Poeciliidae): a histochemical, immunohistochemical, ultrastructural and morphometric study. Anat Embryol 187:353–361
Walpita CN, Crawford AD, Janssens EDR, Van der Geyten S, Darras VM (2009) Type 2 iodothyronine deiodinase is essential for thyroid hormone-dependent embryonic development and pigmentation in zebrafish. Endocrinology 150:530–539
Walpita CN, Crawford AD, Darras VM (2010) Combined antisense knockdown of type 1 and type 2 iodothyronine deiodinases disrupts embryonic development in zebrafish (Danio rerio). Gen Comp Endocrinol 166:134–141
Warshawsky D, Miller L (1995) Tissue-specific in vivo protein-DNA interactions at the promoter region of the Xenopus 63 kDa keratin gene during metamorphosis. Nucleic Acids Res 23:4502–4509
Watanabe Y, Kobayashi H, Suzuki K, Kotani K, Yoshizato K (2001) New epidermal keratin genes from Xenopus laevis: hormonal and regional regulation of their expression during anuran skin metamorphosis. Biochim Biophys Acta 1517:339–350
Watanabe Y, Tanaka R, Kobayashi H, Utoh R, Suzuki K, Obara M, Yoshizato K (2002) Metamorphosis-dependent transcriptional regulation of xak-c, a novel Xenopus type I keratin gene. Dev Dyn 225:561–570
Yamano K, Miwa S, Obinata T, Inui Y (1991) Thyroid hormone regulates developmental changes in muscle during flounder metamorphosis. Gen Comp Endocrinol 81:464–472
Yamano K, Takano-Ohmuro H, Obinata T, Inui Y (1994) Effect of thyroid hormone on developmental transition of myosin light chains during flounder metamorphosis. Gen Comp Endocrinol 93:321–326
Yaoita Y, Brown DD (1990) A correlation of thyroid hormone receptor gene expression with amphibian metamorphosis. Genes Dev 4:1917–1924
Zhang J, Lazar MA (2000) The mechanism of action of thyroid hormones. Annu Rev Physiol 62:439–466
Acknowledgement
We thank Heiddis Smáradóttir of Fiskeldi Eyjafjarðar, IS-600 Akureyri, Iceland, for providing the halibut samples.
Author information
Authors and Affiliations
Corresponding author
Additional information
This project was supported by the European Community project, LIFECYCLE (222719-2) and the Portuguese Ministry of Science (FCT; project PDCT/MAR/115005/2009). M.A.C. was sponsored by the Portuguese Ministry of Science (grant no. SFRH/BPD/66808/2009).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Figure S1
Halibut D1 (a) and D3 (b) cDNA sequences and predicted protein sequences. Double-underlining in a represents the TGA Sec insertion codon. In a, b, single underlining represents the putative polyadenylation signal; sequences in bold represent the SECISearch-predicted SECIS element (star termination codon). SECISearch predicted hhD1 (a’) and hhD3 (b’) SECIS element. Boxed regions in a’ and b’ represent the SECIS core, while bold letters represent conserved SECIS nucleotides. Halibut D2 RT-PCR isolated nucleotide and predicted peptide sequences (c). Double underlining in c represents the TGA Sec insertion codon and boxed amino acids represent characteristic conserved vertebrate D2 amino acids. (JPEG 480 kb)
High Resolution Image 1
(TIFF 11463 kb)
Figure S2
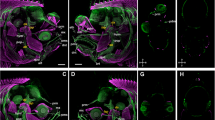
In situ hybridization experiments on halibut larvae sections from stages 5 (a), 7 (b), 9 (c) and 10 (d) hybridized with hhD2 (c, d) or hhD3 (a, b) sense probes. Bar 10 μm. (JPEG 1105 kb)
High Resolution Image 2
(TIFF 51510 kb)
Rights and permissions
About this article
Cite this article
Campinho, M.A., Galay-Burgos, M., Silva, N. et al. Molecular and cellular changes in skin and muscle during metamorphosis of Atlantic halibut (Hippoglossus hippoglossus) are accompanied by changes in deiodinases expression. Cell Tissue Res 350, 333–346 (2012). https://doi.org/10.1007/s00441-012-1473-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-012-1473-x