Abstract
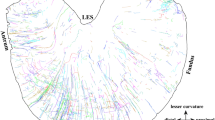
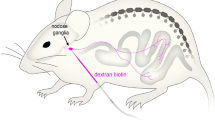
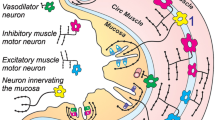
Neural tracers have not typically been employed to determine the pathways followed by axons between their perikarya and target tissues. We have adapted the tetramethylbenzidine method for horseradish peroxidase (HRP) to stain fibers en bloc in organs and thus to delineate axonal trajectories. We have also applied this protocol to characterize the pathways that vagal afferents follow to the intestines. The protocol confirms that the proximal segment of the duodenum receives afferents carried in the vagal hepatic branch and demonstrates that vagal afferents innervating the remainder of the small and large intestines course through multiple fascicles derived from the celiac branches of the abdominal vagus. These fascicles divide, intermingle, and reorganize along the abdominal aorta and superior mesenteric artery (SMA), but not along the inferior mesenteric artery, and then project to the intestines with secondary arteries that branch from the SMA. The inferior pancreaticoduodenal, jejunal, middle colic, right colic, and ileocecocolic arteries all carry vagal afferents to segments of the intestines. As the arteries derived from the SMA divide repeatedly into successively finer branches and course to the intestines, the vagal afferent fascicles (typically a pair) running with each arterial branch also divide. These divisions generate sets/pairs of finer fascicles coursing with even the highest order arterial radicles. The vagal fascicles enter the intestinal wall with the vessels and appear to innervate the organ near the point of entry. The results verify the practicality and sensitivity of the en bloc HRP technique and suggest that the protocol could delineate other peripheral pathways.



Similar content being viewed by others
References
Altschuler SM, Ferenci DA, Lynn RB, Miselis RR (1991) Representation of the cecum in the lateral dorsal motor nucleus of the vagus nerve and commissural subnucleus of the nucleus-tractus-solitarii in rat. J Comp Neurol 304:261–274
Altschuler SM, Escardo J, Lynn RB, Miselis RR (1993) The central organization of the vagus nerve innervating the colon of the rat. Gastroenterology 104:502–509
Baljet B, Drukker J (1979) Extrinsic innervation of the abdominal organs in the female rat. Acta Anat 104:243–267
Baljet B, Groen GJ (1986) Some aspects of the peripheral nervous-system in the human-fetus as revealed by the acetylcholinesterase in toto staining method. Acta Histochem Suppl 32:69–75
Berthoud HR, Powley TL (1992) Vagal afferent innervation of the rat fundic stomach: morphological characterization of the gastric tension receptor. J Comp Neurol 319:261–276
Berthoud H-R, Carlson NR, Powley TL (1991) Topography of efferent vagal innervation of the rat gastrointestinal tract. Am J Physiol 260:R200–R207
Berthoud HR, Kressel M, Raybould HE, Neuhuber WL (1995) Vagal sensors in the rat duodenal mucosa—distribution and structure as revealed by in-vivo DiI-tracing. Anat Embryol 191:203–212
Berthoud HR, Patterson LM, Neumann F, Neuhuber WL (1997) Distribution and structure of vagal afferent intraganglionic laminar endings (IGLEs) in the rat gastrointestinal tract. Anat Embryol 195:183–191
Bloot J, Boekellar AB, Baljet B (1984) A simple acetylcholinesterase method for staining in toto the nervous-system in rat fetuses and a procedure for embedding stained fetuses in transparent epoxy-resin. Stain Tech 59:246–249
Boekellar AB, Baljet B, Drukker J (1985) The arterial vascular supply of the upper abdominal organs in the rat. Neth J Zool 35:455–468
Boekellar AB, Groen GJ, Baljet B (1987) Anatomy of the abdominal branches of the vagus nerves in the rat. Acta Anat 130:12–13
Downman CBB (1952) Distribution along the small intestine of afferent, vasoconstrictor and inhibitory fibres in the mesenteric bundles. J Physiol (Lond) 116:228–235
Fox EA, Powley TL (1985) Longitudinal columnar organization within the dorsal motor nucleus represents separate branches of the abdominal vagus. Brain Res 341:269–282
Hebel R, Stromberg MW (1976) Anatomy of the laboratory rat. Williams & Wilkins, Baltimore, pp 91–111
Holst M-C, Kelly JB, Powley TL (1997) Vagal preganglionic projections to the enteric nervous system characterized with PHA-L. J Comp Neurol 381:81–100
Hopkins DA, Bieger D, deVente J, Steinbuisch HWM (1996) Vagal efferent projections: viscerotopy, neurochemistry and effects of vagotomy. Prog Brain Res 107:79–96
Mesulam M-M (1978) Tetramethyl benzidine for horseradish peroxidase neurohistochemistry: a non-carcinogenic blue reaction-product with superior sensitivity for visualizing neural afferents and efferents. J Histochem Cytochem 26:106–117
Mesulam M-M (1982) Principles of horseradish peroxidase neurohistochemistry and their applications for tracing neural pathways—axonal transport, enzyme histochemistry and light microscopic analysis. In: Mesulam M-M (ed) Tracing neural connections with horseradish peroxidase. Wiley, Chichester, pp 1–151
Morrison JFB (1977) The afferent innervation of the gastrointestinal tract. In: Brooks FP, Evers PW (eds) Nerves and the gut. Slack, New Jersey, pp 297–326
Phillips RJ, Baronowsky EA, Powley TL (1997) Afferent innervation of gastrointestinal tract smooth muscle by the hepatic branch of the vagus. J Comp Neurol 384:248–270
Powley TL, Phillips RJ (2005) Advances in neural tracing of vagal afferent nerves and terminals. In: Undem BJ, Weinreich D (eds) Advances in vagal afferent neurobiology. CRC Press, Boca Raton, pp 123–145
Powley TL, Prechtl JC, Fox EA, Berthoud H-R (1983) Anatomical considerations for surgery of the rat abdominal vagus: distribution, paraganglia and regeneration. J Auton Nerv Syst 9:79–97
Powley TL, Fox EA, Berthoud H-R (1987) Retrograde tracer technique for assessment of selective and total subdiaphragmatic vagotomies. Am J Physiol 253:R361–R370
Prechtl JC, Powley TL (1985) Organization and distribution of the rat subdiaphragmatic vagus and associated paraganglia. J Comp Neurol 235:182–195
Prechtl JC, Powley TL (1987) A light and electron microscopic examination of the vagal hepatic branch of the rat. Anat Embryol 176:115–126
Prechtl JC, Powley TL (1990) The fiber composition of the abdominal vagus of the rat. Anat Embryol 181:101–115
Robertson B, Lindh B, Aldskogius H (1992) WGA-HRP and choleragenoid-HRP as anterogradely transported tracers in vagal visceral afferents and binding of WGA and choleragenoid to nodose ganglion neurons in rodents. Brain Res 590:207–212
Walls EK, Wang FB, Holst M-C, Phillips R, Voreis JS, Perkins AR, Pollard LE, Powley TL (1995) Selective vagal rhizotomies: a new dorsal surgical approach used for intestinal deafferentations. Am J Physiol 269:R1279–R1288
Wang FB, Powley TL (2000) Topographic inventories of vagal afferents in gastrointestinal muscle. J Comp Neurol 421:302–324
Zhang XG, Fogel R, Simpson P, Renehan W (1991) The target specificity of the extrinsic innervation of the rat small-intestine. J Auton Nerv Syst 32:53–62
Zhang XG, Renehan WE, Fogel R (2000) Vagal innervation of the rat duodenum. J Auton Nerv Syst 79:8–18
Acknowledgements
We thank Dr. Robert Phillips for his expert help in the production of the figures.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by grants from the Fulbright-Hays (Fulbright scholarship) and NSC 42114F Exchange Programs and by NSC86-2418-H-194-003-T to F.B.W., and by the National Institute of Diabetes and Digestive and Kidney Diseases, NIH (DK27627 and DK61317) to T.L.P.
The present observations were reported in part at the 2005 annual meeting of the Society for Neuroscience.
Rights and permissions
About this article
Cite this article
Wang, FB., Powley, T.L. Vagal innervation of intestines: afferent pathways mapped with new en bloc horseradish peroxidase adaptation. Cell Tissue Res 329, 221–230 (2007). https://doi.org/10.1007/s00441-007-0413-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-007-0413-7