Abstract
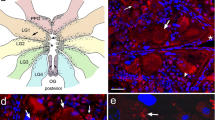
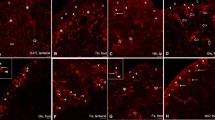
Immunoreactivity to antibodies (ABs) against FMRFamide, CARP, and three FMRFamide gene encoded peptides, i.e., EFLRIamide, the non-FMRFamide peptide "SEEPLY," and the 35-amino-acid "acidic peptide," were investigated in developing embryos and juveniles of Lymnaea stagnalis. Five early transient embryonic neurons revealed immunoreactivity to EFLRIamide. One of the early neurons, the central posterior, also expressed SEEPLY and CARP immunoreactivity. Two neurons in the anlage of the left and right parietal ganglia coexpressed immunoreactivity to EFLRIamide (type 1 transcript) and acidic peptide (type 2 transcript). Within the developing ganglia altogether 30 neurons expressed the type 1 transcript, and three expressed the type 2 transcript. No peripheral cells immunoreactive to SEEPLY or acidic peptide ABs were found, whereas bipolar EFLRIamide- and CARP-immunoreactive cells were abundant in the lip, mantle and foot. After hatching, the number of immunoreactive neurons in ganglia increased up to 223 and the neurons expressing tetrapeptides were dominant (91%). No neurons coexpressing type 1 transcript and type 2 transcript could be detected in juveniles and adults. At this time, an extensive innervation is developed in the periphery, including foot, mantle, buccal mass, salivary glands and alimentary tract, established mainly by EFLRIamide-immunoreactive cells and varicose fibers of extrinsic and intrinsic origin. It is suggested that both sensory and regulatory function can be attributed to the FMRFamide gene encoded tetrapeptides throughout embryonic and juvenile development in Lymnaea, whereas heptapeptides are presumed to play a modulatory role.











Similar content being viewed by others
References
Benjamin PR, Burke JF (1994) Alternative mRNA splicing of the FMRFamide gene and its role in neuropeptidergic signalling in a defined neural network. Bioassays 16:335–342
Benjamin PR, Ings C (1972) Golgi-Cox studies on the central nervous system of a gastropod mollusc. Z Zellforsch 128:564–582
Benjamin PR, Winlow W (1981) The distribution of three wide-acting synaptic inputs to identified neurons in the isolated brain of Lymnaea stagnalis (L.). Comp Biochem Physiol 70A:293–337
Bright K, Kellett E, Saunders SE, Brierley M, Burke JF, Benjamin PR (1993) Mutually exclusive expression of alternatively spliced FMRFamide transcripts in identified neuronal systems of the snail Lymnaea. J Neurosci 13:2719–2729
Brownlee DJA, Fairweather I, Holden-Dye L (1996) Nematode neuropeptides: localization, isolation and functions. Parasitol Today 12:343–351
Croll RP, Chiasson BJ (1989) Postembryonic development of serotoninlike immunoreactivity in the central nervous system of the snail, Lymnaea stagnalis. J Comp Neurol 280:122–142
Croll RP, Voronezhskaya EE (1995) Early FMRFamide-like immunoreactive cells in gastropod neurogenesis. Acta Biol Hung 46:295–303
Croll RP, Voronezhskaya EE (1996) Early elements in gastropod neurogenesis. Dev Biol 173:344–347
Croll RP, Jackson DL, Voronezhskaya EE (1997) Catecholamine-containing cells in larval and postlarval bivalve molluscs. Biol Bull 193:116–124
Croll RP, Voronezhskaya EE, Hiripi L, Elekes K (1999) Development of catecholaminergic neurons in the pond snail, Lymnaea stagnalis: II. Postembryonic development of central and peripheral cells. J Comp Neurol 404:297–307
Dickinson AJG, Croll RP (2001) Neurocalcin-like immunoreactivity in embryonic stages of the gastropod molluscs Aplysia californica and Lymnaea stagnalis. Invert Biol 120:206–216
Dickinson AJG, Nason J, Croll RP (1999) Histochemical localization of FMRFamide, serotonin and catecholamines in embryonic Crepidula fornicata (Gastropoda, Prosobranchia). Zoomorphology 119:49–62
Dickinson AJG, Croll RP, Voronezhskaya EE (2000) Development of embryonic cells containing serotonin, catecholamines and FMRFamide-related peptides in Aplysia californica. Biol Bull 199:305–315
Elekes K, Voronezhskaya EE, Hiripi L, Eckert M, Rapus J (1996) Octopamine in the developing nervous system of the pond snail, Lymnaea stagnalis. Acta Biol Hung 47:73–87
Fujiwara-Sakata M, Muneoka Y, Kobayashi M (1991) Action and immunoreactivity of neuropeptides in the buccal neuromuscular system of a prosobranch mollusc, Rapana thomasiana. Cell Tissue Res 264:57–62
Greenberg MJ, Price DA (1992) Relationships among the FMRFamide-like peptides. In: Joosse J, Buis RM, Tilders FJH (eds) The peptidergic neuron. Progress in brain research. Elsevier, Cambridge, pp 25–37
Hadfield MG (1978) Metamorphosis in marine molluscan larvae: an analysis of stimulus and response. In: Chia FS, Rice M (eds) Settlement and metamorphosis of marine invertebrate larvae. Elsevier, North-Holland, New York, pp 165–175
Hadfield MG, Meleshkevitch EA, Boudko DY (2000) The apical sensory organ of a gastropod veliger is a receptor for settlement cues. Biol Bull 198:67–76
Hernádi L, Terano Y, Muneoka Y, Kiss T (1995) Distribution of catch-relaxing peptide (CARP)-like immunoreactive neurons in the central and peripheral nervous system of Helix pomatia. Cell Tissue Res 280:335–348
Kellett E, Saunders SE, Li KW, Staddon JW, Benjamin PR, Burke JF (1994) Genomic organization of the FMRFamide gene in Lymnaea: multiple exons encoding novel neuropeptides. J Neurosci 14:6564–6570
Kempf SC, Page LR, Pires A (1997) Development of serotonin-like immunoreactivity in the embryos and larvae of nudibranch mollusks with emphasis on the structure and possible function of the apical sensory organ. J Comp Neurol 386:507–528
Marois R, Croll RP (1992) Development of serotonergic cells within the embryonic central nervous system of the pond snail, Lymnaea stagnalis. J Comp Neurol 322:255–265
Mescheriakov VN (1990) The common pond snail Lymnaea stagnalis L. In: Dettlaff DA, Vassetzky SG (eds) Animal species for developmental studies. Plenum, New York, pp 69–132
Nagy T, Elekes K (2002) Ultrastructure of neuromuscular contacts in the embryonic pond snail, Lymnaea stagnalis L. Acta Biol Hung 53:125–139
Santama N, Li KW, Bright K, Yeoman M, Geraerts WPM, Benjamin PR, Burke JF (1993) Processing of the FMRFamide precursor protein in the snail Lymnaea stagnalis: characterization and neuronal localization of a novel peptide, "SEEPLY". Eur J Neurosci 5:1003–1006
Santama N, Wheeler CH, Burke JF, Benjamin PR (1994) Neuropeptides myomodulin, small cardioactive peptides, and buccalin in the central nervous system of Lymnaea stagnalis: purification, immunoreactivity, and artifacts. J Comp Neurol 342:335–351
Santama N, Benjamin PR, Burke JF (1995a) Alternative RNA splicing generates diversity of neuropeptide expression in the brain of the snail Lymnaea: in situ analysis of mutually exclusive transcripts of the FMRFamide gene. Eur J Neurosci 7:65–76
Santama N, Wheeler CH, Skingsley DR, Yeoman MS, Bright K, Kaye I, Burke JF, Benjamin PR (1995b) Identification, distribution and physiological activity of three novel neuropeptides of Lymnaea: EFLRIamide and pQFYRIamide encoded by the FMRFamide gene, and a related peptide. Eur J Neurosci 7:234–246
Santama N, Li KW, Geraerts WPM, Benjamin PR, Burke JF (1996) Post-translational processing of the alternative neuropeptide precursor encoded by the FMRFamide gene in the pulmonate snail Lymnaea stagnalis. Eur J Neurosci 8:968–977
Syed NI, Lukowiak K, Bulloch AGM (1990) In vitro reconstruction of the central pattern generator of the mollusk Lymnaea. Science 250:282–285
Takayanagi H, Takeda N (1988) Dynamics of FMRFamide immunoreactivity in response to physiologically active substances in the central nervous system of the snail, Achatina fulica. Comp Biochem Physiol 91A:609–612
Voronezhskaya EE, Elekes K (1996) Transient and sustained expression of FMRFamide-like immunoreactivity in the developing nervous system of Lymnaea stagnalis (Mollusca, Pulmonata). Cell Mol Neurobiol 16:661–676
Voronezhskaya EE, Pavlova GA, Sakharov DA (1992) Possible control of molluscan embryogenesis by neuronal catecholamines. Russ J Dev Biol 23:295
Voronezhskaya EE, Hiripi L, Elekes K, Croll RP (1999) Development of catecholaminergic neurons in the pond snail, Lymnaea stagnalis: I. Embryonic development of dopamine-containing neurons and dopamine-dependent behaviors. J Comp Neurol 404:297–307
Voronezhskaya EE, Tyurin SA, Nezlin LP (2002) Neuronal development in larval chiton Ischnochiton hakodadensis. J Comp Neurol 444:25–38
Walker RJ (1992) Neuroactive peptides with an RFamide or Famide carboxyl terminal. Comp Biochem Physiol 102C:213–222
Winlow W, Benjamin PR (1976) Neuronal mapping in the brain of the pond snail Lymnaea stagnalis (L.) In: Salánki J (ed) Neurobiology of invertebrates. Gastropoda brain. Akadémiai Kiadó, Budapest, pp 41–59
Worster BM, Yeoman MS, Benjamin PR (1998) Matrix-assisted laser desorption/ionization time flight mass spectrometric analysis of the pattern of peptide expression in single neurons resulting from alternative mRNA splicing of the FMRFamide gene. Eur J Neurosci 10:3498–3507
Acknowledgements
The authors' thanks are due to Professor Paul Benjamin, University of Sussex, for reading and commenting on the paper. The skillful technical assistance of Ms. Éva Karácsonyi and the photographic work of Mr. Boldizsár Balázs are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by grants from the Hungarian Scientific Research Fund (OTKA), Nos. 6284, 23472 and 34106 to K.E., from the Hungarian and Russian Academies of Sciences, exchange program No. 33 to E.E.V. and K.E., and from the Russian Foundation for Basic Research (RFBR), grant 99-04-418 to E.E.V.
Rights and permissions
About this article
Cite this article
Voronezhskaya, E.E., Elekes, K. Expression of FMRFamide gene encoded peptides by identified neurons in embryos and juveniles of the pulmonate snail Lymnaea stagnalis . Cell Tissue Res 314, 297–313 (2003). https://doi.org/10.1007/s00441-003-0800-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-003-0800-7