Abstract
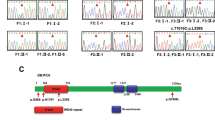
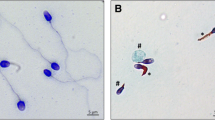
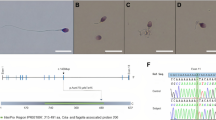
Severe asthenozoospermia is a common cause of male infertility. Recent studies have revealed that SPEF2 mutations lead to multiple morphological abnormalities of the sperm flagella (MMAF) without primary ciliary dyskinesia (PCD) symptoms in males, but PCD phenotype was also found in one female individual. Therefore, whether there is a phenotypic continuum ranging from infertile patients with PCD to MMAF patients with no or low noise PCD manifestations remains elusive. Here, we performed whole-exome sequencing in 47 patients with severe asthenozoospermia from 45 unrelated Chinese families. We identified four novel biallelic mutations in SPEF2 (8.9%, 4/45) in six affected individuals (12.8%, 6/47), while no deleterious biallelic variants in SPEF2 were detected in 637 controls, including 219 with oligoasthenospermia, 195 with non-obstructive azoospermia, and 223 fertile controls. Notably, all six patients exhibited PCD-like symptoms, including recurrent airway infections, bronchitis, and rhinosinusitis. Ultrastructural analysis revealed normal 9 + 2 axonemes of respiratory cilia but consistently abnormal 9 + 0 axoneme or disordered accessory structures of sperm flagella, indicating different roles of SPEF2 in sperm flagella and respiratory cilia. Subsequently, a Spef2 knockout mouse model was used to validate the PCD-like phenotype and male infertility, where the subfertility of female Spef2−/− mice was found unexpectedly. Overall, our data bridge the link between MMAF and PCD based on the association of SPEF2 mutations with both infertility and PCD in males and provide basis for further exploring the molecular mechanism of SPEF2 during spermiogenesis and ciliogenesis.






Similar content being viewed by others
References
Ben Khelifa M, Coutton C, Zouari R, Karaouzene T, Rendu J, Bidart M et al (2014) Mutations in DNAH1, which encodes an inner arm heavy chain dynein, lead to male infertility from multiple morphological abnormalities of the sperm flagella. Am J Hum Genet 94:95–104
Bustamante-Marin XM, Shapiro A, Sears PR, Charng WL, Conrad DF, Leigh MW et al (2019) Identification of genetic variants in CFAP221 as a cause of primary ciliary dyskinesia. J Hum Genet. https://doi.org/10.1038/s10038-019-0686-1
Cindric S, Dougherty GW, Olbrich H, Hjeij R, Loges NT, Amirav I et al (2019) SPEF2- and HYDIN-mutant cilia lack the central pair associated protein SPEF2 aiding PCD diagnostics. Am J Respir Cell Mol Biol. https://doi.org/10.1165/rcmb.2019-0086OC
Coutton C, Vargas AS, Amiri-Yekta A, Kherraf ZE, Ben Mustapha SF, Le Tanno P et al (2018) Mutations in CFAP43 and CFAP44 cause male infertility and flagellum defects in Trypanosoma and human. Nat Commun 9:686
Coutton C, Martinez G, Kherraf ZE, Amiri-Yekta A, Boguenet M, Saut A et al (2019) Bi-allelic mutations in ARMC2 lead to severe astheno-teratozoospermia due to sperm flagellum malformations in humans and mice. Am J Hum Genet 104:331–340
Dyer SJ (2009) International estimates on infertility prevalence and treatment seeking: potential need and demand for medical care. Hum Reprod 24: 2379–2380 (author reply 2380–3)
Edelbusch C, Cindric S, Dougherty GW, Loges NT, Olbrich H, Rivlin J et al (2017) Mutation of serine/threonine protein kinase 36 (STK36) causes primary ciliary dyskinesia with a central pair defect. Hum Mutat 38:964–969
Fassad MR, Shoemark A, le Borgne P, Koll F, Patel M, Dixon M et al (2018) C11orf70 Mutations disrupting the intraflagellar transport-dependent assembly of multiple axonemal dyneins cause primary ciliary dyskinesia. Am J Hum Genet 102:956–972
Guo F, Yang B, Ju ZH, Wang XG, Qi C, Zhang Y et al (2014) Alternative splicing, promoter methylation, and functional SNPs of sperm flagella 2 gene in testis and mature spermatozoa of Holstein bulls. Reproduction 147:241–252
Imtiaz F, Allam R, Ramzan K, Al-Sayed M (2015) Variation in DNAH1 may contribute to primary ciliary dyskinesia. BMC Med Genet 16:14
Krausz C, Riera-Escamilla A (2018) Genetics of male infertility. Nat Rev Urol 15:369–384
Lehti MS, Zhang FP, Kotaja N, Sironen A (2017) SPEF2 functions in microtubule-mediated transport in elongating spermatids to ensure proper male germ cell differentiation. Development 144:2683–2693
Lehti MS, Henriksson H, Rummukainen P, Wang F, Uusitalo-Kylmala L, Kiviranta R et al (2018) Cilia-related protein SPEF2 regulates osteoblast differentiation. Sci Rep 8:859
Leigh MW, Pittman JE, Carson JL, Ferkol TW, Dell SD, Davis SD et al (2009) Clinical and genetic aspects of primary ciliary dyskinesia/Kartagener syndrome. Genet Med 11:473–487
Leigh MW, Hazucha MJ, Chawla KK, Baker BR, Shapiro AJ, Brown DE et al (2013) Standardizing nasal nitric oxide measurement as a test for primary ciliary dyskinesia. Ann Am Thorac Soc 10:574–581
Linck RW, Chemes H, Albertini DF (2016) The axoneme: the propulsive engine of spermatozoa and cilia and associated ciliopathies leading to infertility. J Assist Reprod Genet 33:141–156
Liu C, Lv M, He X, Zhu Y, Amiri-Yekta A, Li W et al (2019a) Homozygous mutations in SPEF2 induce multiple morphological abnormalities of the sperm flagella and male infertility. J Med Genet. https://doi.org/10.1136/jmedgenet-2019-106011
Liu W, Sha Y, Li Y, Mei L, Lin S, Huang X et al (2019b) Loss-of-function mutations in SPEF2 cause multiple morphological abnormalities of the sperm flagella (MMAF). J Med Genet 56:678–684
Loreng TD, Smith EF (2017) The central apparatus of cilia and eukaryotic flagella. Cold Spring Harb Perspect Biol. https://doi.org/10.1101/cshperspect.a028118
Lucas JS, Burgess A, Mitchison HM, Moya E, Williamson M, Hogg C et al (2014) Diagnosis and management of primary ciliary dyskinesia. Arch Dis Child 99:850–856
Lucas JS, Barbato A, Collins SA, Goutaki M, Behan L, Caudri D et al (2017) European Respiratory Society guidelines for the diagnosis of primary ciliary dyskinesia. Eur Respir J. https://doi.org/10.1183/13993003.01090-2016
Merveille AC, Davis EE, Becker-Heck A, Legendre M, Amirav I, Bataille G et al (2011) CCDC39 is required for assembly of inner dynein arms and the dynein regulatory complex and for normal ciliary motility in humans and dogs. Nat Genet 43:72–78
Mirra V, Werner C, Santamaria F (2017) Primary ciliary dyskinesia: an update on clinical aspects, genetics, diagnosis, and future treatment strategies. Front Pediatr 5:135
Mitchell DR, Sale WS (1999) Characterization of a Chlamydomonas insertional mutant that disrupts flagellar central pair microtubule-associated structures. J Cell Biol 144:293–304
Neesen J, Kirschner R, Ochs M, Schmiedl A, Habermann B, Mueller C et al (2001) Disruption of an inner arm dynein heavy chain gene results in asthenozoospermia and reduced ciliary beat frequency. Hum Mol Genet 10:1117–1128
Nsota Mbango JF, Coutton C, Arnoult C, Ray PF, Toure A (2019) Genetic causes of male infertility: snapshot on morphological abnormalities of the sperm flagellum. Basic Clin Androl 29:2
Olbrich H, Schmidts M, Werner C, Onoufriadis A, Loges NT, Raidt J et al (2012) Recessive HYDIN mutations cause primary ciliary dyskinesia without randomization of left-right body asymmetry. Am J Hum Genet 91:672–684
Ostrowski LE, Andrews K, Potdar P, Matsuura H, Jetten A, Nettesheim P (1999) Cloning and characterization of KPL2, a novel gene induced during ciliogenesis of tracheal epithelial cells. Am J Respir Cell Mol Biol 20:675–683
San Agustin JT, Pazour GJ, Witman GB (2015) Intraflagellar transport is essential for mammalian spermiogenesis but is absent in mature sperm. Mol Biol Cell 26:4358–4372
Sanchez-Alvarez J, Cano-Corres R, Fuentes-Arderiu X (2012) A complement for the WHO laboratory manual for the examination and processing of human semen (First Edition, 2010). EJIFCC 23: 103–106
Sha Y, Liu W, Wei X, Zhu X, Luo X, Liang L et al (2019) Biallelic mutations in Sperm flagellum 2 cause human multiple morphological abnormalities of the sperm flagella (MMAF) phenotype. Clin Genet 96:385–393
Sironen A, Kotaja N, Mulhern H, Wyatt TA, Sisson JH, Pavlik JA et al (2011) Loss of SPEF2 function in mice results in spermatogenesis defects and primary ciliary dyskinesia. Biol Reprod 85:690–701
Tan YQ, Tu C, Meng L, Yuan S, Sjaarda C, Luo A et al (2019) Loss-of-function mutations in TDRD7 lead to a rare novel syndrome combining congenital cataract and nonobstructive azoospermia in humans. Genet Med 21:1209–1217
Teves ME, Nagarkatti-Gude DR, Zhang Z, Strauss JF 3rd (2016) Mammalian axoneme central pair complex proteins: Broader roles revealed by gene knockout phenotypes. Cytoskeleton (Hoboken) 73:3–22
Wang H, Yang H, Shivalila CS, Dawlaty MM, Cheng AW, Zhang F et al (2013) One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering. Cell 153:910–918
Wang W, Tu C, Nie H, Meng L, Li Y, Yuan S et al (2019a) Biallelic mutations in CFAP65 lead to severe asthenoteratospermia due to acrosome hypoplasia and flagellum malformations. J Med Genet 56:750–757
Wang WL, Tu CF, Tan YQ (2019b) Insight on multiple morphological abnormalities of sperm flagella in male infertility: what is new? Asian J Androl. https://doi.org/10.4103/aja.aja_53_19
Zhang H, Mitchell DR (2004) Cpc1, a Chlamydomonas central pair protein with an adenylate kinase domain. J Cell Sci 117:4179–4188
Zhao W, Li Z, Ping P, Wang G, Yuan X, Sun F (2018) Outer dense fibers stabilize the axoneme to maintain sperm motility. J Cell Mol Med 22:1755–1768
Zhao L, Hou Y, Picariello T, Craige B, Witman GB (2019) Proteome of the central apparatus of a ciliary axoneme. J Cell Biol 218:2051–2070
Acknowledgements
The authors would like to thank all families and individuals participated in this study. We are grateful to the excellent technical support provided by Junpu Wang, as well as support from the clinical and nursing staff at the Reproductive and Genetic Hospital of CITIC-Xiangya. We also want to thank Director Hong Luo and Dr. Ting Guo in the Department of Respiratory, Second Xiangya Hospital of Central South University for their generous help with PCD diagnosis, and Zhuoyao Guo and Weicheng Chen at the Department of Respiratory, Children's Hospital of Fudan University for their generous help with the high-speed video microscopy for tracheal epithelial cilia and oviduct cilia. We also acknowledge the support of the National Natural Science Foundation of China (81971447 and 81771645 to YQ.T), the National Key Research & Developmental Program of China (2018YFC1004900 to YQ.T), the science and technology major project of the ministry of science and technology of Hunan Province (2017SK1030 to YQ.T), and the China Postdoctoral Science Foundation Funded Project (2019M662786 to CF.T).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
439_2020_2110_MOESM1_ESM.pdf
Figure S1. Analysis for the mutation identified in family 1 and longitudinal ultrastructure of spermatozoa in the affected individuals.a Homozygosity mapping of individuals II-2 and II-3 from family 1. Homozygous regions with a remarkable signal are colored in red. The asterisk indicates the area where SPEF2 is located. b Upper panel: schematic representation of aberrant splicing by skipping SPEF2 exon 17 caused by the variant c.2507+5delG. Lower panel: splice mechanisms of SPEF2 exons associated with the variant c.2507+5delG. The variant leads to a premature termination codon (PTC) in exon 18 (p.A800E fs*3). c TEM photographs of longitudinal sections of SPEF2-mutant individuals. Compared to normal control, the principal piece of mutant sperm flagella (F1: II-3) appears without CPC or RS (red asterisk) and hyperplastic FS (red bracket). Short sperm tails embraced by cytoplasmic residuals (red arrow) with poorly assembled components were observed in the spermatozoa of SPEF2-mutant individuals (F4: II-1). CPC, central pair complex (red arrows); FS, fibrous sheath (blue arrows). Scale bars =1 µm
439_2020_2110_MOESM2_ESM.pdf
Figure S2. TUNEL staining of respiratory epithelia in SPEF2-mutant individual (F4: II-1). TUNEL staining of apoptotic cells in respiratory epithelia of normal control (NC), and SPEF2-mutant individual (F4: II-1). Red arrows indicate apoptotic germ cells (left). Scale bar = 25 μm
439_2020_2110_MOESM3_ESM.pdf
Figure S3. Generation of Spef2-knockout mice. a Gene-targeting construct for Spef2. b Sanger sequencing using PCR products of Spef2−/− mice revealed a large fragment deletion between introns 2-6. c RT-PCR confirmed the lack of exon 4 with primers amplifying exons 1-4, and a shorter product was found with primers amplifying exons 1-9. Subsequently, Sanger sequencing identified a shorter product that lacks exon 3-6. d-e SPEF2 staining is present in the respiratory cilia and whole-length sperm flagella of Spef2+/− mice but absent in that of Spef2−/− mice. Scale bars: (d) 25 μm; (e) 5 μm
439_2020_2110_MOESM4_ESM.pdf
Figure S4. Ultrastructure of spermatozoa in Spef2−/− mice.a-c: TEM photographs of longitudinal-sections of sperms in Spef2+/− (a) and Spef2−/− mice (b-c). Disorganized axoneme or short tails with cytoplasmic residuals containing multiple flagellar components, such as tubulin or fibrous-like elements (asterisk), were observed in the sperm flagella of Spef2−/− mice. Abbreviations: Ax, axoneme; Mi, mitochondria (blue arrow) and FS, fibrous sheath (green arrow). Scale bars for the longitudinal sections =250 nm
439_2020_2110_MOESM5_ESM.pdf
Figure S5. Haploid sperm differentiation was destroyed at stage XI and XII in Spef2−/− mice during spermiogenesis. a H & E staining of cross-sections of testes from Spef2+/− and Spef2−/− mice at 2 months old. A major difference was detected at stages XI and XII during spermiogenesis with a constricted head shape, where sperm tails were absent (asterisk) in the tubular lumen of Spef2−/− mice. Numerous highly condensed nuclei (red arrow) in the seminiferous epithelium at stage IX-XII were observed in Spef2−/− mice, seemingly to be phagocytosed by Sertoli cells. St, Sertoli; Sg, spermatogonia; P, pachytene spermatocyte; RS, round spermatid; ES, elongated spermatid. Scale bar represent 20 μm. b TUNEL staining of apoptotic cells in Spef2+/− and Spef2−/− mice testis sections. Red arrowheads indicate apoptotic germ cells (left). Quantification of apoptotic cells in Spef2+/− and Spef2−/− mice testes (right). Apoptotic cells were counted in 30 random seminiferous tubules. Scale bar = 50 μm. ***p < 0.001
439_2020_2110_MOESM7_ESM.mp4
Movie S1. Analysis of high-speed video microscopy imaging of Spef2+/− mice tracheal epithelial cilia. Cilium beat frequency (CBF) is calculated in beats per second (Hz)
439_2020_2110_MOESM8_ESM.mp4
Movie S2. Analysis of high-speed video microscopy imaging of Spef2−/− mice tracheal epithelial cilia. Cilium beat frequency (CBF) is calculated in beats per second (Hz)
439_2020_2110_MOESM9_ESM.mp4
Movie S3. Analysis of high-speed video microscopy imaging of Spef2+/− mice oviduct cilia. Cilium beat frequency (CBF) is calculated in beats per second (Hz)
439_2020_2110_MOESM10_ESM.mp4
Movie S4. Analysis of high-speed video microscopy imaging of Spef2−/− mice oviduct cilia. Cilium beat frequency (CBF) is calculated in beats per second (Hz)
Rights and permissions
About this article
Cite this article
Tu, C., Nie, H., Meng, L. et al. Novel mutations in SPEF2 causing different defects between flagella and cilia bridge: the phenotypic link between MMAF and PCD. Hum Genet 139, 257–271 (2020). https://doi.org/10.1007/s00439-020-02110-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00439-020-02110-0