Abstract
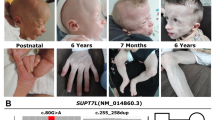
Human Waardenburg syndrome 2A (WS2A) is a dominant hearing loss (HL) syndrome caused by mutations in the microphthalmia-associated transcription factor (MITF) gene. In mouse models with MITF mutations, WS2A is transmitted in a recessive pattern, which limits the study of hearing loss (HL) pathology. In the current study, we performed ENU (ethylnitrosourea) mutagenesis that resulted in substituting a conserved lysine with a serine (p. L247S) in the DNA-binding domain of the MITF gene to generate a novel miniature pig model of WS2A. The heterozygous mutant pig (MITF +/L247S) exhibits a dominant form of profound HL and hypopigmentation in skin, hair, and iris, accompanied by degeneration of stria vascularis (SV), fused hair cells, and the absence of endocochlear potential, which indicate the pathology of human WS2A. Besides hypopigmentation and bilateral HL, the homozygous mutant pig (MITF L247S/L247S) and CRISPR/Cas9-mediated MITF bi-allelic knockout pigs both exhibited anophthalmia. Three WS2 patients carrying MITF mutations adjacent to the corresponding region were also identified. The pig models resemble the clinical symptom and molecular pathology of human WS2A patients perfectly, which will provide new clues for better understanding the etiology and development of novel treatment strategies for human HL.






Similar content being viewed by others
References
Acevedo-Arozena A, Wells S, Potter P, Kelly M, Cox RD, Brown SD (2008) ENU mutagenesis, a way forward to understand gene function. Annu Rev Genom Hum Genet 9:49–69
Aoki H, Moro O (2002) Involvement of microphthalmia-associated transcription factor (MITF) in expression of human melanocortin-1 receptor (MC1R). Life Sci 71:2171–2179
Bauer DC, McMorran BJ, Foote SJ, Burgio G (2015) Genome-wide analysis of chemically induced mutations in mouse in phenotype-driven screens. BMC Genom 16:866
Baxter LL, Pavan WJ (2003) Pmel17 expression is Mitf-dependent and reveals cranial melanoblast migration during murine development. Gene Expr Patterns 3:703–707
Bentley NJ, Eisen T, Goding CR (1994) Melanocyte-specific expression of the human tyrosinase promoter: activation by the microphthalmia gene product and role of the initiator. Mol Cell Biol 14:7996–8006
Capowski EE, Simonett JM, Clark EM et al (2014) Loss of MITF expression during human embryonic stem cell differentiation disrupts retinal pigment epithelium development and optic vesicle cell proliferation. Hum Mol Genet 23:6332–6344
Chen H, Jiang L, Xie Z et al (2010) Novel mutations of PAX3, MITF, and SOX10 genes in Chinese patients with type I or type II Waardenburg syndrome. Biochem Biophys Res Commun 397:70–74
Chen Y, Yang F, Zheng H, Zhu G, Hu P, Wu W (2015) Clinical classification and genetic mutation study of two pedigrees with type II Waardenburg syndrome. Zhonghua Yi Xue Yi Chuan Xue Za Zhi 32:810–813
Chen L, Guo W, Ren L et al (2016) A de novo silencer causes elimination of MITF-M expression and profound hearing loss in pigs. BMC Biol 14:52
Du J, Miller AJ, Widlund HR, Horstmann MA, Ramaswamy S, Fisher DE (2003) MLANA/MART1 and SILV/PMEL17/GP100 are transcriptionally regulated by MITF in melanocytes and melanoma. Am J Pathol 163:333–343
Farrer LA, Grundfast KM, Amos J et al (1992) Waardenburg syndrome (WS) type I is caused by defects at multiple loci, one of which is near ALPP on chromosome 2: first report of the WS consortium. Am J Hum Genet 50:902–913
Geisler R, Rauch GJ, Geiger-Rudolph S et al (2007) Large-scale mapping of mutations affecting zebrafish development. BMC Genom 8:11
Goding CR (2000) Mitf from neural crest to melanoma: signal transduction and transcription in the melanocyte lineage. Genes Dev 14:1712–1728
Guo W, Yi H, Ren L, Chen L, Zhao L, Sun W, Yang SM (2015) The morphology and electrophysiology of the cochlea of the miniature pig. Anat Rec (Hoboken) 298:494–500
Gurr A, Kevenhorster K, Stark T, Pearson M, Dazert S (2010) The common pig: a possible model for teaching ear surgery. Eur Arch Otorhinolaryngol 267:213–217
Hai T, Cao C, Shang H et al (2017) A pilot study of large-scale production of mutant pigs by ENU mutagenesis. eLife 6:e26248
Hauswirth R, Haase B, Blatter M et al (2012) Mutations in MITF and PAX3 cause “splashed white” and other white spotting phenotypes in horses. PLoS Genet 8:e1002653
Hemesath TJ, Steingrimsson E, McGill G et al (1994) Microphthalmia, a critical factor in melanocyte development, defines a discrete transcription factor family. Genes Dev 8:2770–2780
Hodgkinson CA, Moore KJ, Nakayama A, Steingrimsson E, Copeland NG, Jenkins NA, Arnheiter H (1993) Mutations at the mouse microphthalmia locus are associated with defects in a gene encoding a novel basic-helix-loop-helix-zipper protein. Cell 74:395–404
Hrabe de Angelis MH, Flaswinkel H, Fuchs H et al (2000) Genome-wide, large-scale production of mutant mice by ENU mutagenesis. Nat Genet 25:444–447
Hughes MJ, Lingrel JB, Krakowsky JM, Anderson KP (1993) A helix-loop-helix transcription factor-like gene is located at the mi locus. J Biol Chem 268:20687–20690
Hughes AE, Newton VE, Liu XZ, Read AP (1994) A gene for Waardenburg syndrome type 2 maps close to the human homologue of the microphthalmia gene at chromosome 3p12-p14.1. Nat Genet 7:509–512
Kochhar A, Hildebrand MS, Smith RJ (2007) Clinical aspects of hereditary hearing loss. Genet Med 9:393–408
Lekmine F, Chang CK, Sethakorn N, Das Gupta TK, Salti GI (2007) Role of microphthalmia transcription factor (Mitf) in melanoma differentiation. Biochem Biophys Res Commun 354:830–835
Liu XZ, Newton VE, Read AP (1995) Waardenburg syndrome type II: phenotypic findings and diagnostic criteria. Am J Med Genet 55:95–100
Lovell JM, Harper GM (2007) The morphology of the inner ear from the domestic pig (Sus scrofa). J Microsc 228:345–357
Maia AT, Spiteri I, Lee AJ, O’Reilly M, Jones L, Caldas C, Ponder BA (2009) Extent of differential allelic expression of candidate breast cancer genes is similar in blood and breast. Breast Cancer Res 11:R88
Moncini S, Bonati MT, Morella I, Ferrari L, Brambilla R, Riva P (2015) Differential allelic expression of SOS1 and hyperexpression of the activating SOS1 c.755C variant in a Noonan syndrome family. Eur J Hum Genet 23:1531–1537
Mullins MC, Hammerschmidt M, Haffter P, Nusslein-Volhard C (1994) Large-scale mutagenesis in the zebrafish: in search of genes controlling development in a vertebrate. Curr Biol 4:189–202
Nakashima S, Sando I, Takahashi H, Hashida Y (1992) Temporal bone histopathologic findings of Waardenburg’s syndrome: a case report. Laryngoscope 102:563–567
Newton V (1990) Hearing loss and Waardenburg’s syndrome: implications for genetic counselling. J Laryngol Otol 104:97–103
Ni C, Zhang D, Beyer LA et al (2013) Hearing dysfunction in heterozygous Mitf(Mi-wh)/+ mice, a model for Waardenburg syndrome type 2 and Tietz syndrome. Pigment Cell Melanoma Res 26:78–87
Nobukuni Y, Watanabe A, Takeda K, Skarka H, Tachibana M (1996) Analyses of loss-of-function mutations of the MITF gene suggest that haploinsufficiency is a cause of Waardenburg syndrome type 2A. Am J Hum Genet 59:76–83
Nolan PM, Peters J, Strivens M et al (2000) A systematic, genome-wide, phenotype-driven mutagenesis programme for gene function studies in the mouse. Nat Genet 25:440–443
Oliver PL, Davies KE (2012) New insights into behaviour using mouse ENU mutagenesis. Hum Mol Genet 21:R72–R81
Philipp U, Lupp B, Momke S et al (2011) A MITF mutation associated with a dominant white phenotype and bilateral deafness in German Fleckvieh cattle. PLoS One 6:e28857
Pracy JP, White A, Mustafa Y, Smith D, Perry ME (1998) The comparative anatomy of the pig middle ear cavity: a model for middle ear inflammation in the human? J Anat 192(Pt 3):359–368
Read AP, Newton VE (1997) Waardenburg syndrome. J Med Genet 34:656–665
Riley BB, Grunwald DJ (1995) Efficient induction of point mutations allowing recovery of specific locus mutations in zebrafish. Proc Natl Acad Sci USA 92:5997–6001
Russell WL, Kelly EM, Hunsicker PR, Bangham JW, Maddux SC, Phipps EL (1979) Specific-locus test shows ethylnitrosourea to be the most potent mutagen in the mouse. Proc Natl Acad Sci USA 76:5818–5819
Smith SD, Kelley PM, Kenyon JB, Hoover D (2000) Tietz syndrome (hypopigmentation/deafness) caused by mutation of MITF. J Med Genet 37:446–448
Song J, Feng Y, Acke FR, Coucke P, Vleminckx K, Dhooge IJ (2015) Hearing loss in Waardenburg syndrome: a systematic review. Clin Genet 89:416–425
Steingrimsson E, Moore KJ, Lamoreux ML et al (1994) Molecular basis of mouse microphthalmia (mi) mutations helps explain their developmental and phenotypic consequences. Nat Genet 8:256–263
Steingrimsson E, Copeland NG, Jenkins NA (2004) Melanocytes and the microphthalmia transcription factor network. Annu Rev Genet 38:365–411
Stritzel S, Wohlke A, Distl O (2009) A role of the microphthalmia-associated transcription factor in congenital sensorineural deafness and eye pigmentation in Dalmatian dogs. J Anim Breed Genet 126:59–62
Tachibana M, Hara Y, Vyas D, Hodgkinson C, Fex J, Grundfast K, Arnheiter H (1992) Cochlear disorder associated with melanocyte anomaly in mice with a transgenic insertional mutation. Mol Cell Neurosci 3:433–445
Tachibana M, Perez-Jurado LA, Nakayama A et al (1994) Cloning of MITF, the human homolog of the mouse microphthalmia gene and assignment to chromosome 3p14.1-p12.3. Hum Mol Genet 3:553–557
Tachibana M, Kobayashi Y, Matsushima Y (2003) Mouse models for four types of Waardenburg syndrome. Pigment Cell Res 16:448–454
Tassabehji M, Newton VE, Read AP (1994) Waardenburg syndrome type 2 caused by mutations in the human microphthalmia (MITF) gene. Nat Genet 8:251–255
Tassabehji M, Newton VE, Liu XZ et al (1995) The mutational spectrum in Waardenburg syndrome. Hum Mol Genet 4:2131–2137
Vitaterna MH, King DP, Chang AM et al (1994) Mutagenesis and mapping of a mouse gene, Clock, essential for circadian behavior. Science 264:719–725
Waardenburg PJ (1951) A new syndrome combining developmental anomalies of the eyelids, eyebrows and nose root with pigmentary defects of the iris and head hair and with congenital deafness. Am J Hum Genet 3:195–253
Walters EM, Agca Y, Ganjam V, Evans T (2011) Animal models got you puzzled?: think pig. Ann N Y Acad Sci 1245:63–64
Wang X, Zhou J, Cao C et al (2015) Efficient CRISPR/Cas9-mediated biallelic gene disruption and site-specific knockin after rapid selection of highly active sgRNAs in pigs. Sci Rep 5:13348
Wang X, Cao C, Huang J et al (2016) One-step generation of triple gene-targeted pigs using CRISPR/Cas9 system. Sci Rep 6:20620
Wienholds E, van Eeden F, Kosters M, Mudde J, Plasterk RH, Cuppen E (2003) Efficient target-selected mutagenesis in zebrafish. Genome Res 13:2700–2707
Yang S, Dai P, Liu X et al (2013) Genetic and phenotypic heterogeneity in Chinese patients with Waardenburg syndrome type II. PLoS One 8:e77149
Yasumoto K, Yokoyama K, Shibata K, Tomita Y, Shibahara S (1994) Microphthalmia-associated transcription factor as a regulator for melanocyte-specific transcription of the human tyrosinase gene. Mol Cell Biol 14:8058–8070
Yasumoto K, Yokoyama K, Takahashi K, Tomita Y, Shibahara S (1997) Functional analysis of microphthalmia-associated transcription factor in pigment cell-specific transcription of the human tyrosinase family genes. J Biol Chem 272:503–509
Acknowledgements
The authors thank all the personnel at the Beijing Farm Animals Research Center, the Chinese Academy of Sciences, and the Chinese Swine Mutagenesis Consortium, whose names are not listed as co-authors of this paper for their assistance. We appreciate the patients for their invaluable cooperation and participation.
Author information
Authors and Affiliations
Contributions
JZ, SY, and HW conceived the project with input from AM, QZ, and HW. All experiments were performed by TH, WG, JY, AL, MQ, XW, XW, YZ, DW, HS, QH, RZ, QJ, QZ, YL, TZ, and WJ. ZC and CC analyzed the data and conducted the bioinformatics analyses. JZ and JY wrote the manuscript with input from TH, ZC, and WG.
Corresponding authors
Ethics declarations
Funding
This work was supported by the National Natural Science Foundation of China (81671274, 31601008, and 31402045); the Strategic Priority Research Program of CAS (XDA08000000 and XDA01030400); the National Transgenic Project of China (2016ZX08009003-006 and 2014ZX0801007B); the National Basic Research Program of China (2011CBA01005, 2011CB944100, 2011BAI15B02, 2012BAI39B04, and 2012CB967900); the National High Technology Research and Development Program of China (2012AA020602); and the National Institutes of Health (DC006908 to Z-Y C).
Availability of data and material
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Conflict of interest
The authors declare that they have no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hai, T., Guo, W., Yao, J. et al. Creation of miniature pig model of human Waardenburg syndrome type 2A by ENU mutagenesis. Hum Genet 136, 1463–1475 (2017). https://doi.org/10.1007/s00439-017-1851-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00439-017-1851-2