Abstract
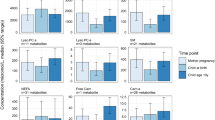
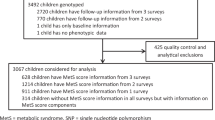
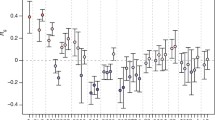
Recent genome-wide association studies of the adult human metabolome have identified genetic variants associated with relative levels of several acylcarnitines, which are important clinical correlates for chronic conditions such as type 2 diabetes and obesity. We have previously shown that these same metabolite levels are highly heritable at birth; however, no studies to our knowledge have examined genetic associations with these metabolites measured at birth. Here, we examine, in 743 newborns, 58 single nucleotide polymorphisms (SNPs) in 11 candidate genes previously associated with differing relative levels of short-chain acylcarnitines in adults. Six SNPs (rs2066938, rs3916, rs3794215, rs555404, rs558314, rs1799958) in the short-chain acyl-CoA dehydrogenase gene (ACADS) were associated with neonatal C4 levels. Most significant was the G allele of rs2066938, which was associated with significantly higher levels of C4 (P = 1.5 × 10−29). This SNP explains 25 % of the variation in neonatal C4 levels, which is similar to the variation previously reported in adult C4 levels. There were also significant (P < 1 × 10−4) associations between neonatal levels of C5-OH and SNPs in the solute carrier family 22 genes (SLC22A4 and SLC22A5) and the 3-methylcrotonyl-CoA carboxylase 1 gene (MCCC1). We have replicated, in newborns, SNP associations between metabolic traits and the ACADS and SLC22A4 genes observed in adults. This research has important implications not only for the identification of rare inborn errors of metabolism but also for personalized medicine and early detection of later life risks for chronic conditions.

Similar content being viewed by others
References
Adams SH, Hoppel CL, Lok KH, Zhao L, Wong SW, Minkler PE et al (2009) Plasma acylcarnitine profiles suggest incomplete long-chain fatty acid beta-oxidation and altered tricarboxylic acid cycle activity in type 2 diabetic African–American women. J Nutr 139(6):1073–1081
Alex S, Lange K, Amolo T, Grinstead JS, Haakonsson AK, Szalowska E et al (2013) Short-chain fatty acids stimulate angiopoietin-like 4 synthesis in human colon adenocarcinoma cells by activating peroxisome proliferator-activated receptor gamma. Mol Cell Biol 33(7):1303–1316
Alul FY, Cook DE, Shchelochkov OA, Fleener LG, Berberich SL, Murray JC et al (2013) The heritability of metabolic profiles in newborn twins. Heredity 110(3):253–258
Atzori L, Antonucci R, Barberini L, Locci E, Marincola FC, Scano P et al (2011) 1H NMR-based metabolomic analysis of urine from preterm and term neonates. Front Biosci 3:1005–1012
Bene J, Komlosi K, Magyari L, Talian G, Horvath K, Gasztonyi B et al (2007) Plasma carnitine ester profiles in Crohn’s disease patients characterized for SLC22A4 C1672T and SLC22A5 G-207C genotypes. Br J Nutr 98(2):345–350
Bene J, Marton M, Mohas M, Bagosi Z, Bujtor Z, Oroszlan T et al (2013) Similarities in serum acylcarnitine patterns in type 1 and type 2 diabetes mellitus and in metabolic syndrome. Ann Nutr Metab 62(1):80–85
Clark RH, Chace DH, Spitzer AR, Pediatrix Amino Acid Study G (2007) Effects of two different doses of amino acid supplementation on growth and blood amino acid levels in premature neonates admitted to the neonatal intensive care unit: a randomized, controlled trial. Pediatrics 120(6):1286–1296
Corydon MJ, Vockley J, Rinaldo P, Rhead WJ, Kjeldsen M, Winter V et al (2001) Role of common gene variations in the molecular pathogenesis of short-chain acyl-CoA dehydrogenase deficiency. Pediatr Res 49(1):18–23
De Preter V, Arijs I, Windey K, Vanhove W, Vermeire S, Schuit F et al (2012) Impaired butyrate oxidation in ulcerative colitis is due to decreased butyrate uptake and a defect in the oxidation pathway. Inflamm Bowel Dis 18(6):1127–1136
Eriksson J, Forsen T, Tuomilehto J, Osmond C, Barker D (2000a) Fetal and childhood growth and hypertension in adult life. Hypertension 36(5):790–794
Eriksson JG, Forsen T, Tuomilehto J, Osmond C, Barker DJ (2000b) Early growth, adult income, and risk of stroke. Stroke 31(4):869–874
Gall WE, Beebe K, Lawton KA, Adam KP, Mitchell MW, Nakhle PJ et al (2010) alpha-Hydroxybutyrate is an early biomarker of insulin resistance and glucose intolerance in a nondiabetic population. PLoS One 5(5):e10883
Gieger C, Geistlinger L, Altmaier E, Hrabe de Angelis M, Kronenberg F, Meitinger T et al (2008) Genetics meets metabolomics: a genome-wide association study of metabolite profiles in human serum. PLoS Genet 4(11):e1000282
Gregersen N, Andresen BS, Corydon MJ, Corydon TJ, Olsen RK, Bolund L et al (2001) Mutation analysis in mitochondrial fatty acid oxidation defects: exemplified by acyl-CoA dehydrogenase deficiencies, with special focus on genotype-phenotype relationship. Hum Mutat 18(3):169–189
Hornbak M, Banasik K, Justesen JM, Krarup NT, Sandholt CH, Andersson A et al (2011) The minor C-allele of rs2014355 in ACADS is associated with reduced insulin release following an oral glucose load. BMC Med Genet 12:4
Illig T, Gieger C, Zhai G, Romisch-Margl W, Wang-Sattler R, Prehn C et al (2010) A genome-wide perspective of genetic variation in human metabolism. Nat Genet 42(2):137–141
Jung CW, Lee BH, Kim JH, Kim GH, Lee J, Choi JH et al (2012) Uneventful clinical courses of Korean patients with methylcrotonylglycinuria and their common mutations. J Hum Genet 57(1):62–64
Kelleher AS, Clark RH, Steinbach M, Chace DH, Spitzer AR, Pediatrix Amino-Acid Study G (2008) The influence of amino-acid supplementation, gestational age and time on thyroxine levels in premature neonates. J Perinatol 28(4):270–274
Kelley DE, Goodpaster B, Wing RR, Simoneau JA (1999) Skeletal muscle fatty acid metabolism in association with insulin resistance, obesity, and weight loss. Am J Physiol 277(6 Pt 1):E1130–E1141
la Marca G, Malvagia S, Toni S, Piccini B, Di Ciommo V, Bottazzo GF (2013) Children who develop type 1 diabetes early in life show low levels of carnitine and amino acids at birth: does this finding shed light on the etiopathogenesis of the disease? Nutr Diabetes 3:e94
Lapillonne A, Griffin IJ (2013) Feeding preterm infants today for later metabolic and cardiovascular outcomes. J Pediatr 162(3 Suppl):S7–S16
Lewandowski AJ, Bradlow WM, Augustine D, Davis EF, Francis J, Singhal A et al (2013) Right ventricular systolic dysfunction in young adults born preterm. Circulation 128(7):713–720
Lowe WL Jr, Bain JR (2013) “Prediction is very hard, especially about the future”: new biomarkers for type 2 diabetes? Diabetes 62(5):1384–1385
Mathai S, Derraik JG, Cutfield WS, Dalziel SR, Harding JE, Biggs J et al (2013) Increased adiposity in adults born preterm and their children. PLoS One 8(11):e81840
Nagan N, Kruckeberg KE, Tauscher AL, Bailey KS, Rinaldo P, Matern D (2003) The frequency of short-chain acyl-CoA dehydrogenase gene variants in the US population and correlation with the C(4)-acylcarnitine concentration in newborn blood spots. Mol Genet Metab 78(4):239–246
Parkinson JR, Hyde MJ, Gale C, Santhakumaran S, Modi N (2013) Preterm birth and the metabolic syndrome in adult life: a systematic review and meta-analysis. Pediatrics 131(4):e1240–e1263
Pasquali M, Monsen G, Richardson L, Alston M, Longo N (2006) Biochemical findings in common inborn errors of metabolism. Am J Med Genet C Semin Med Genet 142C(2):64–76
Pedersen CB, Bischoff C, Christensen E, Simonsen H, Lund AM, Young SP et al (2006) Variations in IBD (ACAD8) in children with elevated C4-carnitine detected by tandem mass spectrometry newborn screening. Pediatr Res 60(3):315–320
Ryckman KK, Berberich SL, Shchelochkov OA, Cook DE, Murray JC (2013) Clinical and environmental influences on metabolic biomarkers collected for newborn screening. Clin Biochem 46(1–2):133–138
Schooneman MG, Vaz FM, Houten SM, Soeters MR (2013) Acylcarnitines: reflecting or inflicting insulin resistance? Diabetes 62(1):1–8
Shah SH, Hauser ER, Bain JR, Muehlbauer MJ, Haynes C, Stevens RD et al (2009) High heritability of metabolomic profiles in families burdened with premature cardiovascular disease. Mol Syst Biol 5:258
Shibbani K, Fahed A, Al-Shaar L, Arabi M, Nemer G, Bitar F et al (2013) Primary carnitine deficiency: novel mutations and insights into the cardiac phenotype. Clin Genet 85(2):127–137
Suhre K, Shin SY, Petersen AK, Mohney RP, Meredith D, Wagele B et al (2011) Human metabolic individuality in biomedical and pharmaceutical research. Nature 477(7362):54–60
Yeh CS, Wang JY, Cheng TL, Juan CH, Wu CH, Lin SR (2006) Fatty acid metabolism pathway play an important role in carcinogenesis of human colorectal cancers by microarray-bioinformatics analysis. Cancer Lett 233(2):297–308
Acknowledgments
We express our thanks to Kim Piper at the Iowa Department of Public Health and the members of the Congenital and Inherited Disorders Advisory Committee for their enthusiastic support and management of this project. We thank Teresa Snell, Franklin Delin and Dariush Shirazi from the State Hygienic Laboratory for their assistance in the acquisition of the newborn screening data and samples. We thank Sara Copeland at the Health Resources Services Administration for her guidance on this project. Research reported in this publication was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health under Award Number R00HD065786. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of interest
The authors declare they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Data: Two tables of additional results are included in the supplementary data word document files.
Rights and permissions
About this article
Cite this article
Ryckman, K.K., Smith, C.J., Jelliffe-Pawlowski, L.L. et al. Metabolic heritability at birth: implications for chronic disease research. Hum Genet 133, 1049–1057 (2014). https://doi.org/10.1007/s00439-014-1450-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00439-014-1450-4