Abstract
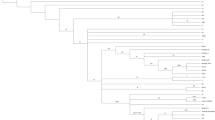
We report here the analyses of complete mtDNA coding region sequences from more than 270 Alzheimer’s disease (AD) patients and normal controls to determine if inherited mtDNA mutations contribute to the etiology of AD. The AD patients and normal individuals were carefully screened and drawn from two populations of European descent in an effort to avoid spurious effects due to local population anomalies. Overall, there were no significant haplogroup associations in the combined AD and normal control sequence sets. Reduced median network analysis revealed that the AD mtDNA sequences contained a higher number of substitutions in tRNA genes, and that there was an elevated frequency of replacement substitutions in the complex I genes of the control sequences. Analysis of the replacement substitutions indicated that those arising in the AD mtDNAs were no more deleterious, on average, than those in the control mtDNAs. The only evidence for the synergistic action of mutations was the presence of both a rare non-conservative replacement substitution and a tRNA mutation in 2 AD mtDNAs, from a total of 145, whereas such a combination of mutations was not observed in the control sequences. Overall, the results reported here indicate that pathogenic inherited mtDNA mutations do not constitute a major etiological factor in sporadic AD. At most, a small proportion of AD patients carry a pathogenic mtDNA mutation and a small proportion of cognitively normal aged individuals carry a mtDNA mutation that reduces the risk of AD.


Similar content being viewed by others
References
Anandatheerthavarada HK, Biswas G, Robin M-A, Avadhani NG (2003) Mitochondrial targeting and a novel transmembrane arrest of Alzheimer’s amyloid precursor protein impairs mitochondrial function in neuronal cells. J Cell Biol 161:41–54
Andrews RM, Kubacka I, Chinnery PF, Lightowlers RN, Turnbull DM, Howell N (1999) Reanalysis and revision of the Cambridge reference sequence for human mitochondrial DNA. Nat Genet 23:147
Bandelt H-J, Forster P, Sykes BC, Richards MB (1995) Mitochondrial portraits of human populations using median networks. Genetics 141:743–753
Beal MF (2005) Mitochondria take center stage in aging and neurodegeneration. Ann Neurol 58:495–505
Bosetti F, Brizzi F, Barogi S, Mancuso M, Siciliano G, Tendi EA, Murri L, Rapoport SI, Solaini G (2002) Cytochrome c oxidase and mitochondrial F1F0-ATPase (ATPase) activities in platelets and brain from patients with Alzheimer’s disease. Neurobiol Aging 23:371–376
Brown MD, Yang C-C, Trounce I, Torroni A, Lott MT, Wallace DC (1992) A mitochondrial DNA variant, identified in Leber hereditary optic neuropathy patients, which extends the amino acid sequence of cytochrome c oxidase subunit I. Am J Hum Genet 51:378–385
Busciglio J, Pelsman A, Wong C, Pigino G, Yuan M, Mori H, Yankner BA (2002) Altered metabolism of the amyloid β precursor protein is associated with mitochondrial dysfunction in Down’s syndrome. Neuron 33:677–688
Cardoso SM, Proenca MT, Santos S, Santana I, Oliveira CR (2004) Cytochrome c oxidase is decreased in Alzheimer’s disease platelets. Neurobiol Aging 25:105–110
Casley CS, Land JM, Sharpe MA, Clark JB, Duchen MR, Canevari L (2002) β amyloid fragment 25–35 causes mitochondrial dysfunction in primary cortical neurons. Neurobiol Dis 10:258–267
Castellani R, Hirai K, Aliev G, Drew KL, Nunomura A, Takeda A, Cash AD, Obrenovich ME, Perry G, Smith MA (2002) Role of mitochondrial dysfunction in Alzheimer’s disease. J Neurosci Res 70:357–360
Chagnon P, Betard C, Robitaille Y, Cholette A, Gauvreau D (1995) Distribution of cytochrome oxidase activity in various neurodegenerative diseases. Mol Neurosci 6:711–715
Chagnon P, Gee M, Filion M, Robitaille Y, Belouchi M, Gauvreau D (1999) Phylogenetic analyses of the mitochondrial genome indicates significant differences between patients with Alzheimer disease and controls in a French–Canadian founder population. Am J Med Genet 85:20–30
Chinnery PF, Johnson M, Taylor RW, Lightowlers RN, Turnbull DM (1997) A novel mitochondrial tRNA phenylalanine gene mutation presenting with acute rhabdomyolysis. Ann Neurol 41:408–410
Chinnery PF, Taylor GA, Howell N, Andrews RM, Morris CM, Taylor RW, McKeith IG, Perry RH, Edwardson JA, Turnbull DM (2000) Mitochondrial DNA haplogroups and susceptibility to AD and dementia with Lewy bodies. Neurol 55:302–304
Chinnery PF, Taylor GA, Howell N, Brown DT, Parsons TJ, Turnbull DM (2001) Point mutations of the mtDNA control region in normal and neurodegenerative human brains. Am J Hum Genet 68:529–532
Coskun PE, Beal MF, Wallace DC (2004) Alzheimer’s brains harbor somatic mtDNA control-region mutations that suppress mitochondrial transcription and replication. Proc Natl Acad Sci USA 101:10726–10731
Cottrell DA, Blakely EL, Johnson MA, Ince PG, Turnbull DM (2001) Mitochondrial enzyme-deficient hippocampal neurons and choroidal cells in AD. Neurology 57:260–264
Cottrell DA, Borthwick GM, Johnson MA, Ince PG, Turnbull DM (2002) The role of cytochrome c oxidase deficient hippocampal neurons in Alzheimer’s disease. J Neuropath Appl Neurobiol 28:390–396
Crouch PJ, Blake R, Duce JA, Ciccotosto GD, Li Q-X, Barnham KJ, Curtain CC, Cherny RA, Cappai R, Dyrks T, Masters CL, Trounce IA (2005) Copper-dependent inhibition of human cytochrome c oxidase by a dimeric conformer of amyloid-β1–42. J Neurosci 25:672–679
Danielson SR, Carelli V, Tan G, Martinuzzi A, Schapira AHV, Savontaus M-L, Cortopassi GA (2005) Isolation of transcriptomal changes attributable to LHON mutations and the cybridization process. Brain 128:1026–1037
DiMauro S, Schon E (2001) Mitochondrial DNA mutations in human disease, Am J Med Genet 106:18–26
Elson JL, Turnbull DM, Howell N (2004) Comparative genomics and the evolution of human mitochondrial DNA: assessing the effects of selection. Am J Hum Genet 74:229–238
Ghosh SS, Swerdlow RH, Miller SW, Sheeman B, Parker WD, Davis RE (1999) Use of cytoplasmic hybrid lines for elucidating the role of mitochondrial dysfunction in Alzheimer’ disease and Parkinson’s disease. Ann NY Acad Sci 893:176–191
Gonnet GH, Cohen MA, Benner SA (1992) Exhaustive matching of the entire protein sequence database. Science 256:1443–1445
Herrnstadt C, Howell N (2004) An evolutionary perspective on pathogenic mtDNA mutations: haplogroup associations of clinical disorders. Mitochondrion 4:791–798
Herrnstadt C, Elson JL, Fahy E, Preston G, Turnbull DM, Anderson C, Ghosh SS, Olefsky JM, Beal MF, Davis RE, Howell N (2002) Reduced-median-network analysis of complete mitochondrial DNA coding-region sequences for the major African, Asian, and European haplogroups. Am J Hum Genet 70:1152–1171
Herrnstadt C, Preston G, Howell N (2003) Errors, phantom and otherwise, in human mtDNA sequences. Am J Hum Genet 72:1585–1586
Hirai K, Aliev G, Nunomura A, Fujioka H, Russell RL, Atwood CS, Johnson AB, Kress Y, Vinters HV, Tabaton M, Shimohama S, Cash AD, Siedlak SL, Harris PLR, Jones PK, Petersen RB, Perry G, Smith MA (2001) Mitochondrial abnormalities in Alzheimer’s disease. J Neurosci 21:3017–3023
Howell N, Oostra R-J, Bolhuis PA, Spruijt L, Clarke LA, Mackey DA, Preston G, Herrnstadt C (2003a) Sequence analysis of the mitochondrial genomes from Dutch pedigrees with Leber hereditary optic neuropathy. Am J Hum Genet 72:1460–1469
Howell N, Smejkal CB, Mackey DA, Chinnery PF, Turnbull DM, Herrnstadt C (2003b) The pedigree rate of sequence divergence in the human mitochondrial genome: there is a difference between phylogenetic and pedigree rates. Am J Hum Genet 72:659–670
Howell N, Elson JL, Chinnery PF, Turnbull DM (2005) mtDNA mutations and common neurodegenerative disorders. Trends Genet 21:583–584
Ioannidis JPA (2005) Why most published research findings are false. PloS Med 2:e124
Ito S, Ohta S, Nishimaki K, Kagawa Y, Soma R, Kuno S, Komatsuzaki Y, Mizusawa H, Hayashi J-I (1999) Functional integrity of mitochondrial genomes in human platelets and autopsied brain tissues from elderly patients with Alzheimer’s disease. Proc Natl Acad Sci USA 96:2099–2103
Keers SM, Gibson AM, Turnbull DM, Chinnery PF (2004) No evidence of an association between the mtDNA 16184-93 polyC tract and late onset dementia. J Med Genet 41:957–958
Khan SM, Cassarino DS, Abramova NN, Keeney PM, Borland MK, Trimmer PA, Krebs CT, Bennett JC, Parks JK, Swerdlow RH, Parker WD, Bennett JP (2000) Alzheimer’s disease cybrids replicate β-amyloid abnormalities through cell death pathways. Ann Neurol 48:148–155
Kish SJ, Bergeran C, Rajput A, Dozie S, Mastrogiacomo F, Chang LJ, Wilson JM, DiStefano LM, Nobrega JN (1992) Brain cytochrome oxidase activity is reduced in Alzheimer’s disease. J Neurochem 59:776–779
Kish SJ, Mastrogiacomo F, Guttman M, Furukawa Y, Taanman J-W, Dozic S, Pandolfo M, Lamarche J, DiStefano L, Chang L-J (1999) Decreased brain protein levels of cytochrome oxidase subunits in Alzheimer’s disease and in hereditary spincerebellar ataxia disorders: a nonspecific change? J Neurochem 72:700–707
Kondrashov FA (2005) Prediction of pathogenic mutations in mitochondrially encoded human tRNAs. Hum Mol Genet 14:2415–2419
Lehtonen MS, Moilanen JS, Majamaa K (2003) Increased variation in mtDNA in patients with familial sensorineural hearing impairment. Hum Genet 113:220–227
Lin MT, Simon DK, Ahn CG, Kim LM, Beal MF (2002) High aggregate burden of somatic mtDNA point mutations in aging and Alzheimer’s disease brain. Hum Mol Genet 11:133–145
Maurer I, Zierz S, Moller H-J (2000) A selective defect of cytochrome c oxidase is present in brain of Alzheimer’s disease patients. Neurobiol Aging 21:455–462
Mayeux R (2003) Epidemiology of neurodegeneration. Ann Rev Neurosci 26:81–104
McFarland R, Elson JL, Taylow RW, Howell N, Turnbull DM (2004a) Assigning pathogenicity to mitochondrial tRNA mutations: when “definitely maybe” is not good enough. Trends Genet 20:591–596
McFarland R, Taylor RW, Chinnery PF, Howell N, Turnbull DM (2004b) A novel sporadic mutation in cytochrome c oxidase subunit II as a cause of rhabdomyolysis. Neuromuscul Disord 14:162–166
Moilanen JS, Majamaa K (2003) Phylogenetic network and physiochemical properties of nonsynonymous mutations in the protein-coding genes of human mitochondrial DNA. Mol Biol Evol 20:1195–1210
Mutisya EM, Bowling AC, Beal MF (1994) Cortical cytochrome oxidase activity is reduced in Alzheimer’s disease. J Neurochem 63:2179–2184
Niemi A-K, Hervonen A, Hurme M, Karhunen PJ, Jylhä M, Majamaa K (2003) Mitochondrial DNA polymorphisms associated with longevity in a Finnish population. Hum Genet 112:29–33
Ng PC, Henkoff JG, Henikoff S (2000) PHAT: a transmembrane-specific substitution matrix. Bioinformatics 16:760–766
Parker WD, Parks JK (2005) Mitochondrial ND5 mutations in idiopathic Parkinson’s disease. Biochem Biophys Res Commun 326:667–669
Parker WD, Filley CM, Parks JK (1990) Cytochrome oxidase deficiency in Alzheimer’s disease. Neurol 40:1302–1303
Parker WD, Parks JK, Filley CM, Kleinschmidt-Demasters BK (1994) Electron transport chain defects in Alzheimer’s disease brain. Neurol 44:1090–1096
Persson B, Argos P (1994) Prediction of transmembrane segments in proteins utilizing multiple sequence alignments. J Mol Biol 237:182–192
Richards M, Macaulay V, Hickey E, Vega E, Sykes B, Guida V, Rengo C, Sellitto D, Cruciani F, Kivisild T, Villems R, Thomas M, Rychkov S, Rychkov O, Rychkov Y, Golge M, Dimitrov D, Hill E, Bradley D, Romano V, Cali F, Vona G, Demaine A, Papiha S, Triantaphyidis C, Stefanescu G, Hatina J, Belledi M, Di renzo A, Novelletto A, Oppenheim A, Norby S, Al-Zaheri N, Santachiara-Benerecetti S, Scozzari R, Torroni A, Bandelt H-J (2000) Tracing European founder lineages in the Near Eastern mtDNA pool. Am J Hum Genet 67:1251–1277
Rose G, Passarino G, Carrieri G, Altomare K, Greco V, Bertolini S, Bonafe M, Franceschi C, De Benedictis G (2001) Paradoxes in longevity: sequence analysis of mtDNA haplogroup J in centenarians. Eur J Hum Genet 9:701–707
Roubertoux PL, Sluyter F, Carlier M, Marcet B, Maarouf-Veray F, Cherif C, Marcian C, Arrechi P, Godin F, Jamon M, Verrier B, Cohen-Salmon C (2003) Mitochondrial DNA modifies cognition in interaction with the nuclear genome and age in mice. Nat Genet 35:65–69
Schon EA, Manfredi G (2003) Neuronal degeneration and mitochondrial dysfunction. J Clin Invest 111:303–312
Schon EA, Shoubridge EA, Moraes CT (1998) Cybrids in Alzheimer’s disease: a cellular model of the disease? Neurology 51:326–327
Simonian NA, Hyman BT (1993) Functional alterations in Alzheimer’s disease: diminution of cytochrome oxidase in the hippocampal formation. J Neuropath Exp Neurol 52:580–585
Simonian NA, Hyman BT (1994) Functional alterations in Alzheimer’s disease: selective loss of mitochondrial-encoded cytochrome oxidase mRNA in the hippocampal formation. J Neuropath Exp Neurol 53:508–512
Smith MA, Drew KL, Nunomara A, Takeda A, Hirai K, Zhu X, Atwood CS, Raina AK, Rottkamp CA, Sayre LM, Friedland RP, Perry G (2002) Amyloid-β, tau alterations and mitochondrial dysfunction in Alzheimer disease: the chickens or the eggs? Neurochem Int 40:527–531
Swerdlow RH, Parks JK, Cassarino DS, Maguire DJ, Maguire RS, Bennett JPJ, Davis RE, Parker WD (1997) Cybrids in Alzheimer’s disease: a cellular model of the disease? Neurology 49:918–925
Thomas PD, Kejariwal A (2004) Coding single-nucleotide polymorphisms associated with complex vs. Mendelian disease: evolutionary evidence for differences in molecular effects. Proc Natl Acad Sci USA 101:15398–15403
Trimmer PA, Swerdlow RH Parks JK, Keeney P, Bennett JP, Miller SW, Davis RE, Parker WD (2000) Abnormal mitochondrial morphology in sporadic Parkinson’s and Alzheimer’s disease cybrid cell lines. Exp Neurol 162:37–50
Trimmer PA, Keeney PM, Borland MK, Simon FA, Almeida J, Swerdlow R, Parks JP, Parker WD, Bennett JP (2004) Mitochondrial abnormalities in cybrid cell models of sporadic Alzheimer’s disease worsen with passage in culture. Neurobiol Dis 15:29–39
Valla J, Berndt JD, Gonzalez-Lima F (2001) Energy hypometabolism in posterior cingulate cortex of Alzheimer’s patients: superficial laminar cytochrome oxidase associated with disease duration. J Neurosci 21:4923–4930
Vilmi T, Moilanen JS, Finnila S, Majamaa K (2005) Sequence variation in the tRNA genes of human mitochondrial DNA. J Mol Evol 60:587–597
van der Walt JM, Dementieva YA, Martin ER, Scott WK, Nicodemus KK, Kroner CC, Welsh-Bohmer KA, Saunders AM, Roses AD, Small GW, Schmechel DE, Doraisamy PM, Gilbert JR, Haines JL, Vance JM, Pericak-Vance MA (2004) Analysis of European mitochondrial haplogroups with Alzheimer disease risk. Neurosci Lett 365:28–32
Zhu X, Raina AK, Perry G, Smith MA (2004) Alzheimer’s disease: the two-hit hypothesis. Lancet Neurol 3:219–226
Acknowledgements
We thank Dr. H. Payami (University of Oregon Health Science University) for providing some of the samples used for the present study. Dr. Eoin Fahy (MitoKor Inc.) assisted with data storage and analysis. This research was supported by a Wellcome Collaboration Grant (to D. M. T., N. H.), an MRC BioInformatics Fellowship (J. L. E.), and grant AGO P50-5131 from the National Institutes of Health (L. T.).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Elson, J.L., Herrnstadt, C., Preston, G. et al. Does the mitochondrial genome play a role in the etiology of Alzheimer’s disease?. Hum Genet 119, 241–254 (2006). https://doi.org/10.1007/s00439-005-0123-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00439-005-0123-8