Abstract
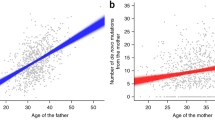
De novo dominant mutations in the GFAP gene have recently been associated with nearly all cases of Alexander disease, a rare but devastating neurological disorder. These heterozygous mutations must occur very early in development and be present in nearly all cells in order to be detected by the sequencing methods used. To investigate whether the mutations may have arisen in the parental germ lines, we determined the parental chromosome bearing the mutations for 28 independent Alexander disease cases. These cases included 17 different missense mutations and one insertion mutation. To enable assignment of the chromosomal origin of the mutations, six new single nucleotide polymorphisms in the GFAP gene were identified, bringing the known total to 26. In 24 of the 28 cases analyzed, the paternal chromosome carried the GFAP mutation (P<0.001), suggesting that they predominantly arose in the parental germ line, with most occurring during spermatogenesis. No effect of paternal age was observed. There has been considerable debate about the magnitude of the male to female germ line mutation rate; our ratio of 6:1 is consistent with indirect estimates based on the rate of evolution of the sex chromosome relative to the autosomic chromosomes.

Similar content being viewed by others
References
Brenner M, Johnson AB, Boespflug-Tanguy O, Rodriguez D, Goldman JE, Messing A (2001) Mutations in GFAP, encoding glial fibrillary acidic protein, are associated with Alexander disease. Nat Genet 27:117–120
Cronn R, Cedroni M, Haselkorn T, Grover C, Wendel JF (2002) PCR-mediated recombination in amplification products derived from polyploid cotton. Theor Appl Genet 104:482–489
Crow JF (2000) The origins, patterns and implications of human spontaneous mutation. Nat Rev Genet 1:40–47
den Dunnen JT, Antonarakis SE (2001) Nomenclature for the description of human sequence variations. Hum Genet 109:121–124
Glaser RL, Jiang W, Boyadjiev SA, Tran AK, Zachary AA, Van Maldergem L, Johnson D, Walsh S, Oldridge M, Wall SA, Wilkie AO, Jabs EW (2000) Paternal origin of FGFR2 mutations in sporadic cases of Crouzon syndrome and Pfeiffer syndrome. Am J Hum Genet 66:768–777
Goriely A, McVean GA, Rojmyr M, Ingemarsson B, Wilkie AO (2003) Evidence for selective advantage of pathogenic FGFR2 mutations in the male germ line. Science 301:643–646
Gorospe JR, Naidu S, Johnson AB, Puri V, Raymond GV, Jenkins SD, Pedersen RC, Lewis D, Knowles P, Fernandez R, De Vivo D, van der Knaap MS, Messing A, Brenner M, Hoffman EP (2002) Molecular findings in symptomatic and pre-symptomatic Alexander disease patients. Neurology 58:1494–1500
Grimm T, Meng G, Liechti-Gallati S, Bettecken T, Muller CR, Muller B (1994) On the origin of deletions and point mutations in Duchenne muscular dystrophy: most deletions arise in oogenesis and most point mutations result from events in spermatogenesis. J Med Genet 31:183–186
Hurst LD, Ellegren H (1998) Sex biases in the mutation rate. Trends Genet 14:446–452
Isaacs A, Baker M, Hutton M (1998) Determination of the gene structure of human GFAP and absence of coding region mutations associated with frontatemporal dementia with Parkinsonism linked to chromosome 17. Genomics 51:152–154
Iwaki T, Kume-Iwaki A, Liem RK, Goldman JE (1989) Alpha B-crystallin is expressed in non-lenticular tissues and accumulates in Alexander’s disease brain. Cell 57:71–78
Iwaki T, Iwaki A, Tateishi J, Sakaki Y, Goldman JE (1993) Alpha B-crystallin and 27-kd heat shock protein are regulated by stress conditions in the central nervous system and accumulate in Rosenthal fibers. Am J Pathol 143:487–495
Jadayel D, Fain P, Upadhyaya M, Ponder MA, Huson SM, Carey J, Fryer A, Mathew CG, Barker DF, Ponder BA (1990) Paternal origin of new mutations in von Recklinghausen neurofibromatosis. Nature 343:558–559
Johnson AB, Bettica A (1989) On-grid immunogold labeling of glial intermediate filaments in epoxy-embedded tissue. Am J Anat 185:335–341
Kinoshita T, Imaizumi T, Miura Y, Fujimoto H, Ayabe M, Shoji H, Okamoto Y, Takashima H, Osame M, Nakagawa M (2003) A case of adult-onset Alexander disease with Arg416Trp human glial fibrillary acidic protein gene mutation. Neurosci Lett 350:169–172
Li R, Messing A, Goldman JE, Brenner M (2002a) GFAP mutations in Alexander disease. Int J Dev Neurosci 20:259–268
Li WH, Yi S, Makova K (2002b) Male-driven evolution. Curr Opin Genet Dev 12:650–656
Li R, Johnson AB, Salomons G, Goldman JE, Naidu S, Quinlan R, Cree B, Ruyle SZ, Banwell B, D’Hooghe M, Siebert JR, Rolf CM, Cox H, Reddy A, Gutie´rrez-Solana LG, Collins A, Weller RO, Messing A, van der Knaap MS, Brenner M (2005) Glial fibrillary acidic protein mutations in infantile, juvenile, and adult forms of Alexander disease. Ann Neurol 57:310–326
Makova KD, Li WH (2002) Strong male-driven evolution of DNA sequences in humans and apes. Nature 416:624–626
Messing A, Goldman JE, Johnson AB, Brenner M (2001) Alexander disease: new insights from genetics. J Neuropathol Exp Neurol 60:563–573
Mignot C, Boespflug-Tanguy O, Gelot A, Dautigny A, Pham-Dinh D, Rodriguez D (2004) Alexander disease: putative mechanisms of an astrocytic encephalopathy. Cell Mol Life Sci 61:369–385
Monk M (1995) Epigenetic programming of differential gene expression in development and evolution. Dev Genet 17:188–197
Namekawa M, Takiyama Y, Aoki Y, Takayashiki N, Sakoe K, Shimazaki H, Taguchi T, Tanaka Y, Nishizawa M, Saito K, Matsubara Y, Nakano I (2002) Identification of GFAP gene mutation in hereditary adult-onset Alexander’s disease. Ann Neurol 52:779–785
Nobuhara Y, Nakahara K, Higuchi I, Yoshida T, Fushiki S, Osame M, Arimura K, Nakagawa M (2004) Juvenile form of Alexander disease with GFAP mutation and mitochondrial abnormality. Neurology 63:1302–1304
Risch N, Reich EW, Wishnick MM, McCarthy JG (1987) Spontaneous mutation and parental age in humans. Am J Hum Genet 41:218–248
Robertson KD, Wolffe AP (2000) DNA methylation in health and disease. Nat Rev Genet 1:11–19
Rodriguez D, Gauthier F, Bertini E, Bugiani M, Brenner M, N’guyen S, Goizet C, Gelot A, Surtees R, Pedespan JM, Hernandorena X, Troncoso M, Uziel G, Messing A, Ponsot G, Pham-Dinh D, Dautigny A, Boespflug-Tanguy O (2001) Infantile Alexander disease: spectrum of GFAP mutations and genotype–phenotype correlation. Am J Hum Genet 69:1134–1140
Sawaishi Y, Yano T, Takaku I, Takada G (2002) Juvenile Alexander disease with a novel mutation in glial fibrillary acidic protein gene. Neurology 58:1541–1543
Singh R, Nielsen AL, Johansen MG, Jorgensen AL (2003) Genetic polymorphism and sequence evolution of an alternatively spliced exon of the glial fibrillary acidic protein gene, GFAP. Genomics 82:185–193
Tartaglia M, Cordeddu V, Chang H, Shaw A, Kalidas K, Crosby A, Patton MA, Sorcini M, van der Burgt I, Jeffery S, Gelb BD (2004) Paternal germline origin and sex-ratio distortion in transmission of PTPN11 mutations in Noonan syndrome. Am J Hum Genet 75:492–497
Tomokane N, Iwaki T, Tateishi J, Iwaki A, Goldman JE (1991) Rosenthal fibers share epitopes with alpha B-crystallin, glial fibrillary acidic protein, and ubiquitin, but not with vimentin. Immunoelectron microscopy with colloidal gold. Am J Pathol 138:875–885
Trappe R, Laccone F, Cobilanschi J, Meins M, Huppke P, Hanefeld F, Engel W (2001) MECP2 mutations in sporadic cases of Rett syndrome are almost exclusively of paternal origin. Am J Hum Genet 68:1093–1101
van der Knaap MS, Salomons GS, Li R, Franzoni E, Gutierrez-Solana LG, Smit LM, Robinson R, Ferrie CD, Cree B, Reddy A, Thomas N, Banwell B, Barkhof F, Jakobs C, Johnson A, Messing A, Brenner M (2005) Unusual variants of Alexander’s disease. Ann Neurol 57:327–338
Wilkin DJ, Szabo JK, Cameron R, Henderson S, Bellus GA, Mack ML, Kaitila I, Loughlin J, Munnich A, Sykes B, Bonaventure J, Francomano CA (1998) Mutations in fibroblast growth-factor receptor 3 in sporadic cases of achondroplasia occur exclusively on the paternally derived chromosome. Am J Hum Genet 63:711–716
Zlotogora J (1998) Germ line mosaicism. Hum Genet 102:381–386
Acknowledgements
We are indebted to the patients and their families for participating in this study. We thank Robecca Anderson for technical assistance in all phases of this research. Human tissues and case information were graciously provided by Drs. Enrico Bertini, Barbara Burton, Amanda Collins, Marc D’Hooghe, Luis González Gutiérrez-Solana, Alyssa Reddy, Cristin M. Rolf and Joseph Siebert; and also were obtained from the NICHD Brain and Tissue Bank for Developmental Disorders under NICHD contracts N01-HD-43368 and NO1-HD-8–3284; from the National Neurological Research Specimen Bank, VAMC, Los Angeles, CA 90073, sponsored by NINDS/NIMH, the National Multiple Sclerosis Society, the Hereditary Disease Foundation, and the Veterans Health Services and Research Administration, Department of Veterans Affairs; and from the Laboratorio di Diagnosi Pre e Postnatale delle Malattie Metaboliche (Instituto G. Gaslini; supported by Telethon project C.20). We also thank the AECOM Human Genetics Program for preparation of lymphoblasts and DNA samples, the UAB MMRC Molecular Biology Core for recombinant DNA support, and the UAB Genomics Core Facility of the Howell and Elizabeth Heflin Center for Human Genetics for DNA sequencing. MB, JEG and AM were supported by NINDS grant P01NS42803; MB was also supported by MRRC grant P30HD38985 and the Lei Foundation, and AM by MRRC grant P30HD03352; ABJ was supported in part by the United Leukodystrophy Foundation; DR was supported in part by the European Leukodystrophy Association (ELA) and the Fédération des Maladies Orphelines (FMO).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, R., Johnson, A.B., Salomons, G.S. et al. Propensity for paternal inheritance of de novo mutations in Alexander disease. Hum Genet 119, 137–144 (2006). https://doi.org/10.1007/s00439-005-0116-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00439-005-0116-7