Abstract
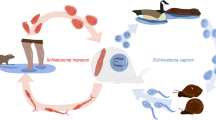
Inducible defences against parasites that are only activated when needed can mitigate the cost of immune or behavioural evasion of parasites. Priming of the immune system and activation of behavioural defences can follow exposure to cues associated with imminent infection risk. In contrast, prior infection can cause immune depression or leave the host with less energy to defend itself against further infections. We investigate the priming of anti-parasite defences and the effect of prior infections in the amphipod Paracalliope fluviatilis, the second intermediate host of the trematode Coitocaecum parvum. During experimental infections, amphipods that had been primed by exposure to chemical cues (from first intermediate snail hosts infected by C. parvum) of infection risk were not better at avoiding further infection than control amphipods. All amphipods showed the same swimming behaviour, whether or not they had been primed by chemical cues from infected snails, or whether or not they were in the presence of live infective stages. In contrast, regardless of whether or not they had been exposed to control water or chemical cues from infected snails, amphipods harbouring prior infections acquired in nature were significantly more likely to acquire new parasites under controlled conditions. These results suggest that the induction of defences via external cues associated with the threat of infection do not play a role in the amphipod’s anti-parasite strategy. However, prior infections may pre-dispose a host to acquire further parasites, with consequences for the distribution of parasites among host individuals and the regulation of the host population.



Similar content being viewed by others
References
Anderson RM, Gordon DM (1982) Processes influencing the distribution of parasite numbers within host populations with special emphasis on parasite-induced host mortalities. Parasitology 85:373–398
Bates AE, Poulin R, Lamare MD (2010) Spatial variation in parasite-induced mortality in an amphipod: shore height versus exposure history. Oecologia 163:651–659
Bates D, Maechler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stats Software 67:1–48
Bryan-Walker K, Leung TLF, Poulin R (2007) Local adaptation of immunity against a trematode parasite in marine amphipod populations. Mar Biol 152:687–695
Christensen RHB (2015) Ordinal: regression models for ordinal data. R package version 2015.6–28 http://www.cran.r-project.org/package=ordinal
Cornet S, Biard C, Moret Y (2009a) Variation in immune defence among populations of Gammarus pulex (Crustacea: Amphipoda). Oecologia 159:257–269
Cornet S, Franceschi N, Bauer A, Rigaud T, Moret Y (2009b) Immune depression induced by acanthocephalan parasites in their intermediate crustacean host: consequences for the risk of super-infection and links with host behavioural manipulation. Int J Parasitol 39:221–229
Fredensborg BL, Mouritsen KN, Poulin R (2004) Intensity-dependent mortality of Paracalliope novizealandiae (Amphipoda: Crustacea) infected by a trematode: experimental infections and field observations. J Exp Mar Biol Ecol 311:253–265
Fredensborg BL, Mouritsen KN, Poulin R (2005) Impact of trematodes on host survival and population density in the intertidal gastropod Zeacumantus subcarinatus. Mar Ecol Progr Ser 290:109–117
Friesen OC, Poulin R, Lague C (2017) Differential impacts of shared parasites on fitness components among competing hosts. Ecol Evol 7:4682–4693
Galaktionov KV, Dobrovolskij AA (2003) The biology and evolution of trematodes. Kluwer Academic Publishers, Dordrecht
Hart BL (1994) Behavioural defences against parasites: interaction with parasite invasiveness. Parasitology 109:S139–S151
Harvell CD (1990) The ecology and evolution of inducible defences. Q Rev Biol 65:323–340
Hazlett BA (2010) Chemical cues and reducing the risk of predation. In: Breithaupt T, Thiel M (eds) Chemical communication in crustaceans. Springer, New York, pp 355–370
James C, Noyes K, Stumbo A, Wisenden B, Goater C (2008) Cost of exposure to trematode cercariae and learned recognition and avoidance of parasitism risk by fathead minnows Pimephales promelas. J Fish Biol 73:2238–2248
Karvonen A, Seppälä O, Valtonen ET (2004a) Parasite resistance and avoidance behaviour in preventing eye fluke infections in fish. Parasitology 129:159–164
Karvonen A, Hudson PJ, Seppälä O, Valtonen ET (2004b) Transmission dynamics of a trematode parasite: exposure, acquired resistance and parasite aggregation. Parasitol Res 92:183–188
Kats LB, Dill LM (1998) The scent of death: chemosensory assessment of predation risk by prey animals. Ecoscience 5:361–394
Kiesecker JM, Skelly DK (2000) Choice of oviposition site by gray treefrogs: the role of potential parasitic infection. Ecology 81:2939–2943
Kurtz J, Franz K (2003) Innate defence: evidence for memory in invertebrate immunity. Nature 425:37–38
Lagrue C, Poulin R (2007) Life cycle abbreviation in the trematode Coitocaecum parvum: can parasites adjust to variable conditions? J Evol Biol 20:1189–1195
Lagrue C, Poulin R (2015) Bottom-up regulation of parasite population densities in freshwater ecosystems. Oikos 124:1639–1647
Leung TLF, Keeney DB, Poulin R (2010) Genetics, intensity-dependence, and host manipulation in the trematode Curtuteria australis: following the strategy of others? Oikos 119:393–400
Litman GW, Rast JP, Fugmann SD (2010) The origins of vertebrate adaptive immunity. Nat Rev Immunol 10:543–553
Lochmiller RL, Deerenberg C (2000) Trade-offs in evolutionary immunology: just what is the cost of immunity? Oikos 88:87–98
Masri L, Cremer S (2014) Individual and social immunisation in insects. Trends Immunol 35:471–482
Moret Y, Schmid-Hempel P (2000) Survival for immunity: the price of immune system activation for bumblebee workers. Science 290:1166–1168
Mothersill C, Austin D, Fernandez-Palomo C, Seymour C, Auchinachie N, Austin B (2015) Rescue of fish exposed to a lethal dose of pathogen by signals from sublethally exposed survivors. FEMS Microbiol Lett 362:fnu058
Noldus LPJJ, Spink AJ, Tegelenbosch RAJ (2001) EthoVision: a versatile video tracking system for automation of behavioral experiments. Behav Res Methods Instr Comp 33:398–414
Norval RAI, Andrew HR, Yunker CE (1989) Pheromone mediation of host-selection in bont ticks (Amblyomma hebraeum Koch). Science 243:364–365
Osnas EE, Lively CM (2005) Immune response to sympatric and allopatric parasites in a snail-trematode interaction. Front Zool 2:8
Osnas EE, Lively CM (2006) Host ploidy, parasitism and immune defence in a coevolutionary snail-trematode system. J Evol Biol 19:42–48
Poulin R (2013) Explaining variability in parasite aggregation levels among host samples. Parasitology 140:541–546
Poulin R, Rau ME, Curtis MA (1991) Infection of brook trout fry, Salvelinus fontinalis, by ectoparasitic copepods: the role of host behaviour and initial parasite load. Anim Behav 41:467–476
Poulin R, Marcogliese DJ, McLaughlin JD (1999) Skin-penetrating parasites and the release of alarm substances in juvenile rainbow trout. J Fish Biol 55:47–53
R Core Team (2017) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Rauque C, Paterson R, Poulin R, Tompkins DM (2011) Do different parasite species interact in their effects on host fitness? A case study on parasites of the amphipod Paracalliope fluviatilis. Parasitology 138:1176–1182
Rohr JR, Swan A, Raffel TR, Hudson PJ (2009) Parasites, info-disruption, and the ecology of fear. Oecologia 159:447–454
Schaller M, Miller GE, Gervais WM, Yager S, Chen E (2010) Mere visual perception of other people’s disease symptoms facilitates a more aggressive immune response. Psychol Sci 21:649–652
Sharp JG, Garnick S, Elgar MA, Coulson G (2015) Parasite and predator risk assessment: nuanced use of olfactory cues. Proc R Soc B 282:20151941
Shaw DJ, Dobson AP (1995) Patterns of macroparasite abundance and aggregation in wildlife populations: a quantitative review. Parasitology 111:S111–S133
Sheldon BC, Verhulst S (1996) Ecological immunology: costly parasite defences and trade-offs in evolutionary ecology. Trends Ecol Evol 11:317–321
Shudo E, Iwasa Y (2001) Inducible defense against pathogens and parasites: optimal choice among multiple options. J Theor Biol 209:233–247
Stevenson RJ, Hodgson D, Oaten MJ, Barouei J, Case TI (2011) The effect of disgust on oral immune function. Psychophysiology 48:900–907
Thomas F, Renaud F, Rousset F, Cézilly F, De Meeus T (1995) Differential mortality of two closely related host species induced by one parasite. Proc R Soc B 260:349–352
Acknowledgements
We thank Sheri Johnson and Anne Besson for providing access to EthoVision. CS was supported by a postdoctoral fellowship from the German Research Foundation (DFG, SE 2728/1-1).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
McPherson, O.G., Friesen, O.C., Selbach, C. et al. Prior infections or defence priming: what determines the risk of trematode infections in amphipod hosts?. Parasitol Res 117, 1915–1923 (2018). https://doi.org/10.1007/s00436-018-5885-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-018-5885-8